Mitochondrial Cytochrome c Oxidase Biogenesis Is Regulated by the Redox State of a Heme-Binding Translational Activator
- PMID: 26415097
- PMCID: PMC4761835
- DOI: 10.1089/ars.2015.6429
Mitochondrial Cytochrome c Oxidase Biogenesis Is Regulated by the Redox State of a Heme-Binding Translational Activator
Abstract
Aim: Mitochondrial cytochrome c oxidase (COX), the last enzyme of the respiratory chain, catalyzes the reduction of oxygen to water and therefore is essential for cell function and viability. COX is a multimeric complex, whose biogenesis is extensively regulated. One type of control targets cytochrome c oxidase subunit 1 (Cox1), a key COX enzymatic core subunit translated on mitochondrial ribosomes. In Saccharomyces cerevisiae, Cox1 synthesis and COX assembly are coordinated through a negative feedback regulatory loop. This coordination is mediated by Mss51, a heme-sensing COX1 mRNA-specific processing factor and translational activator that is also a Cox1 chaperone. In this study, we investigated whether Mss51 hemylation and Mss51-mediated Cox1 synthesis are both modulated by the reduction-oxidation (redox) environment.
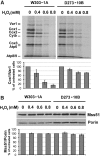
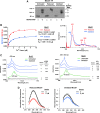
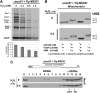
Results: We report that Cox1 synthesis is attenuated under oxidative stress conditions and have identified one of the underlying mechanisms. We show that in vitro and in vivo exposure to hydrogen peroxide induces the formation of a disulfide bond in Mss51 involving CPX motif heme-coordinating cysteines. Mss51 oxidation results in a heme ligand switch, thereby lowering heme-binding affinity and promoting its release. We demonstrate that in addition to affecting Mss51-dependent heme sensing, oxidative stress compromises Mss51 roles in COX1 mRNA processing and translation.
Innovation: H2O2-induced downregulation of mitochondrial translation has so far not been reported. We show that high H2O2 concentrations induce a global attenuation effect, but milder concentrations specifically affect COX1 mRNA processing and translation in an Mss51-dependent manner.
Conclusion: The redox environment modulates Mss51 functions, which are essential for regulation of COX biogenesis and aerobic energy production.
Figures






Similar articles
-
Cox25 teams up with Mss51, Ssc1, and Cox14 to regulate mitochondrial cytochrome c oxidase subunit 1 expression and assembly in Saccharomyces cerevisiae.J Biol Chem. 2011 Jan 7;286(1):555-66. doi: 10.1074/jbc.M110.188805. Epub 2010 Nov 10. J Biol Chem. 2011. PMID: 21068384 Free PMC article.
-
A heme-sensing mechanism in the translational regulation of mitochondrial cytochrome c oxidase biogenesis.Cell Metab. 2012 Dec 5;16(6):801-13. doi: 10.1016/j.cmet.2012.10.018. Cell Metab. 2012. PMID: 23217259 Free PMC article.
-
The Cox1 C-terminal domain is a central regulator of cytochrome c oxidase biogenesis in yeast mitochondria.J Biol Chem. 2017 Jun 30;292(26):10912-10925. doi: 10.1074/jbc.M116.773077. Epub 2017 May 10. J Biol Chem. 2017. PMID: 28490636 Free PMC article.
-
Cytochrome c oxidase biogenesis - from translation to early assembly of the core subunit COX1.FEBS Lett. 2023 Jun;597(12):1569-1578. doi: 10.1002/1873-3468.14671. Epub 2023 May 31. FEBS Lett. 2023. PMID: 37247261 Review.
-
Mitochondrial cytochrome c oxidase biogenesis: Recent developments.Semin Cell Dev Biol. 2018 Apr;76:163-178. doi: 10.1016/j.semcdb.2017.08.055. Epub 2017 Sep 8. Semin Cell Dev Biol. 2018. PMID: 28870773 Free PMC article. Review.
Cited by
-
BOLA3 and NFU1 link mitoribosome iron-sulfur cluster assembly to multiple mitochondrial dysfunctions syndrome.Nucleic Acids Res. 2023 Nov 27;51(21):11797-11812. doi: 10.1093/nar/gkad842. Nucleic Acids Res. 2023. PMID: 37823603 Free PMC article.
-
A Novel Function of Pet54 in Regulation of Cox1 Synthesis in Saccharomyces cerevisiae Mitochondria.J Biol Chem. 2016 Apr 22;291(17):9343-55. doi: 10.1074/jbc.M116.721985. Epub 2016 Feb 29. J Biol Chem. 2016. PMID: 26929411 Free PMC article.
-
The Complexity of Mitochondrial Complex IV: An Update of Cytochrome c Oxidase Biogenesis in Plants.Int J Mol Sci. 2018 Feb 27;19(3):662. doi: 10.3390/ijms19030662. Int J Mol Sci. 2018. PMID: 29495437 Free PMC article. Review.
-
MrpL35, a mitospecific component of mitoribosomes, plays a key role in cytochrome c oxidase assembly.Mol Biol Cell. 2017 Nov 15;28(24):3489-3499. doi: 10.1091/mbc.E17-04-0239. Epub 2017 Sep 20. Mol Biol Cell. 2017. PMID: 28931599 Free PMC article.
-
Coordination of metal center biogenesis in human cytochrome c oxidase.Nat Commun. 2022 Jun 24;13(1):3615. doi: 10.1038/s41467-022-31413-1. Nat Commun. 2022. PMID: 35750769 Free PMC article.
References
-
- Arlt H, Tauer R, Feldmann H, Neupert W, and Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell 85: 875–885, 1996 - PubMed
-
- Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, and Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 86: 960–966, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

