Inhibiting ERK Activation with CI-1040 Leads to Compensatory Upregulation of Alternate MAPKs and Plasminogen Activator Inhibitor-1 following Subtotal Nephrectomy with No Impact on Kidney Fibrosis
- PMID: 26415098
- PMCID: PMC4586140
- DOI: 10.1371/journal.pone.0137321
Inhibiting ERK Activation with CI-1040 Leads to Compensatory Upregulation of Alternate MAPKs and Plasminogen Activator Inhibitor-1 following Subtotal Nephrectomy with No Impact on Kidney Fibrosis
Abstract
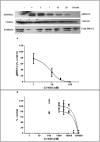
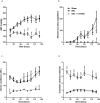
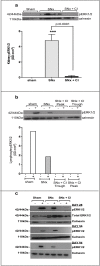
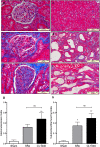
Extracellular-signal regulated kinase (ERK) activation by MEK plays a key role in many of the cellular processes that underlie progressive kidney fibrosis including cell proliferation, apoptosis and transforming growth factor β1-mediated epithelial to mesenchymal transition. We therefore assessed the therapeutic impact of ERK1/2 inhibition using a MEK inhibitor in the rat 5/6 subtotal nephrectomy (SNx) model of kidney fibrosis. There was a twentyfold upregulation in phospho-ERK1/2 expression in the kidney after SNx in Male Wistar rats. Rats undergoing SNx became hypertensive, proteinuric and developed progressive kidney failure with reduced creatinine clearance. Treatment with the MEK inhibitor, CI-1040 abolished phospho- ERK1/2 expression in kidney tissue and prevented phospho-ERK1/2 expression in peripheral lymphocytes during the entire course of therapy. CI-1040 had no impact on creatinine clearance, proteinuria, glomerular and tubular fibrosis, and α-smooth muscle actin expression. However, inhibition of ERK1/2 activation led to significant compensatory upregulation of the MAP kinases, p38 and JNK in kidney tissue. CI-1040 also increased the expression of plasminogen activator inhibitor-1 (PAI-1), a key inhibitor of plasmin-dependent matrix metalloproteinases. Thus inhibition of ERK1/2 activation has no therapeutic effect on kidney fibrosis in SNx possibly due to increased compensatory activation of the p38 and JNK signalling pathways with subsequent upregulation of PAI-1.
Conflict of interest statement
Figures



Similar articles
-
Urokinase-induced smooth muscle cell responses require distinct signaling pathways: a role for the epidermal growth factor receptor.J Vasc Surg. 2005 Apr;41(4):672-81. doi: 10.1016/j.jvs.2005.01.007. J Vasc Surg. 2005. PMID: 15874933
-
Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals.J Am Soc Nephrol. 2007 Mar;18(3):846-59. doi: 10.1681/ASN.2006080886. Epub 2007 Jan 31. J Am Soc Nephrol. 2007. PMID: 17267741
-
Reduction of chronic allograft nephropathy by inhibition of extracellular signal-regulated kinase 1 and 2 signaling.Am J Physiol Renal Physiol. 2008 Sep;295(3):F672-9. doi: 10.1152/ajprenal.90285.2008. Epub 2008 Jul 9. Am J Physiol Renal Physiol. 2008. PMID: 18614619
-
Plasminogen activator inhibitor type 1 is a potential target in renal fibrogenesis.Kidney Int. 2000 Nov;58(5):1841-50. doi: 10.1111/j.1523-1755.2000.00355.x. Kidney Int. 2000. PMID: 11044203 Review.
-
[Molecular mechanisms of nephro-protective action of enalapril in experimental chronic renal failure].Ann Acad Med Stetin. 1999;Suppl 52:1-93. Ann Acad Med Stetin. 1999. PMID: 10589103 Review. Polish.
Cited by
-
Cell Division Cycle 42 Improves Renal Functions, Fibrosis, Th1/Th17 Infiltration and Inflammation to Some Degree in Diabetic Nephropathy.Inflammation. 2025 Aug;48(4):1987-1997. doi: 10.1007/s10753-024-02169-1. Epub 2024 Nov 13. Inflammation. 2025. PMID: 39535664
-
MEK-inhibitors decrease Nfix in muscular dystrophy but induce unexpected calcifications, partially rescued with Cyanidin diet.iScience. 2023 Dec 10;27(1):108696. doi: 10.1016/j.isci.2023.108696. eCollection 2024 Jan 19. iScience. 2023. PMID: 38205246 Free PMC article.
-
ELABELA-derived peptide ELA13 attenuates kidney fibrosis by inhibiting the Smad and ERK signaling pathways.J Zhejiang Univ Sci B. 2024 Apr 15;25(4):341-353. doi: 10.1631/jzus.B2300033. J Zhejiang Univ Sci B. 2024. PMID: 38584095 Free PMC article.
-
Profiling Transcriptional Regulation and Functional Roles of Schistosoma mansoni c-Jun N-Terminal Kinase.Front Genet. 2019 Oct 18;10:1036. doi: 10.3389/fgene.2019.01036. eCollection 2019. Front Genet. 2019. PMID: 31681440 Free PMC article.
References
-
- Bokemeyer D, Panek D, Kramer HJ, Lindemann M, Kitahara M, Boor P, et al. In vivo identification of the mitogen-activated protein kinase cascade as a central pathogenic pathway in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2002;13(6):1473–80. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

