Clinical Relevance of Biomarkers of Oxidative Stress
- PMID: 26415143
- PMCID: PMC4657513
- DOI: 10.1089/ars.2015.6317
Clinical Relevance of Biomarkers of Oxidative Stress
Abstract
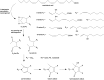
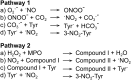
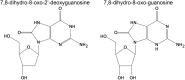
Significance: Oxidative stress is considered to be an important component of various diseases. A vast number of methods have been developed and used in virtually all diseases to measure the extent and nature of oxidative stress, ranging from oxidation of DNA to proteins, lipids, and free amino acids.
Recent advances: An increased understanding of the biology behind diseases and redox biology has led to more specific and sensitive tools to measure oxidative stress markers, which are very diverse and sometimes very low in abundance.
Critical issues: The literature is very heterogeneous. It is often difficult to draw general conclusions on the significance of oxidative stress biomarkers, as only in a limited proportion of diseases have a range of different biomarkers been used, and different biomarkers have been used to study different diseases. In addition, biomarkers are often measured using nonspecific methods, while specific methodologies are often too sophisticated or laborious for routine clinical use.
Future directions: Several markers of oxidative stress still represent a viable biomarker opportunity for clinical use. However, positive findings with currently used biomarkers still need to be validated in larger sample sizes and compared with current clinical standards to establish them as clinical diagnostics. It is important to realize that oxidative stress is a nuanced phenomenon that is difficult to characterize, and one biomarker is not necessarily better than others. The vast diversity in oxidative stress between diseases and conditions has to be taken into account when selecting the most appropriate biomarker.
Figures







References
-
- Abbasi A, Corpeleijn E, Gansevoort RT, Gans ROB, Struck J, Schulte J, Hillege HL, van der Harst P, Stolk RP, Navis G, and Bakker SJL. Circulating peroxiredoxin 4 and type 2 diabetes risk: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Diabetologia 57: 1842–1849, 2014 - PMC - PubMed
-
- Ahrens I, Habersberger J, Baumlin N, Qian H, Smith BK, Stasch J-P, Bode C, Schmidt HHHW, and Peter K. Measuring oxidative burden and predicting pharmacological response in coronary artery disease patients with a novel direct activator of haem-free/oxidised sGC. Atherosclerosis 218: 431–434, 2011 - PubMed
-
- Alvarez B. and Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25: 295–311, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
