BARD1 mediates TGF-β signaling in pulmonary fibrosis
- PMID: 26415510
- PMCID: PMC4587901
- DOI: 10.1186/s12931-015-0278-3
BARD1 mediates TGF-β signaling in pulmonary fibrosis
Abstract
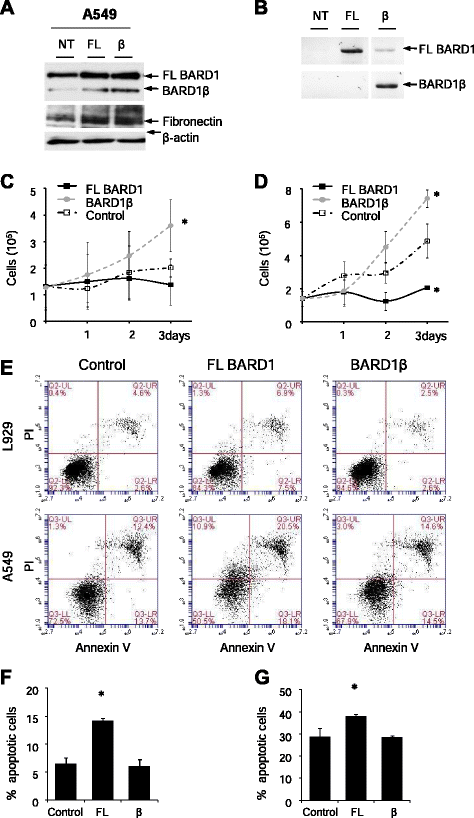
Background: Idiopathic pulmonary fibrosis (IPF) is a rapid progressive fibro-proliferative disorder with poor prognosis similar to lung cancer. The pathogenesis of IPF is uncertain, but loss of epithelial cells and fibroblast proliferation are thought to be central processes. Previous reports have shown that BARD1 expression is upregulated in response to hypoxia and associated with TGF-β signaling, both recognized factors driving lung fibrosis. Differentially spliced BARD1 isoforms, in particular BARD1β, are oncogenic drivers of proliferation in cancers of various origins. We therefore hypothesized that BARD1 and/or its isoforms might play a role in lung fibrosis.
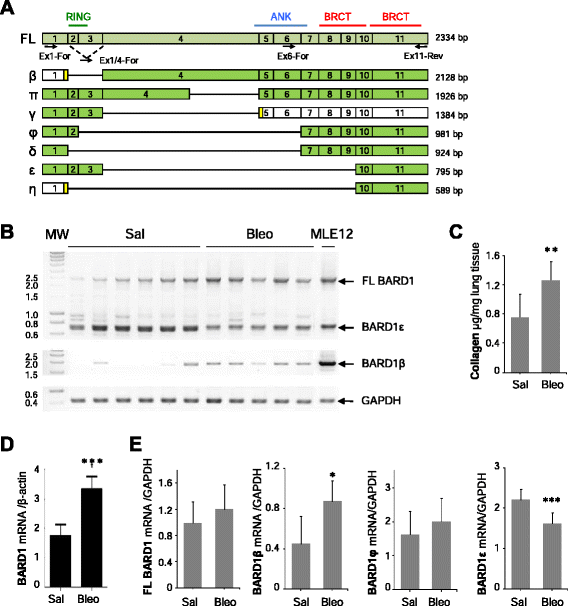
Methods: We investigated BARD1 expression as a function of TGF-β in cultured cells, in mice with experimentally induced lung fibrosis, and in lung biopsies from pulmonary fibrosis patients.
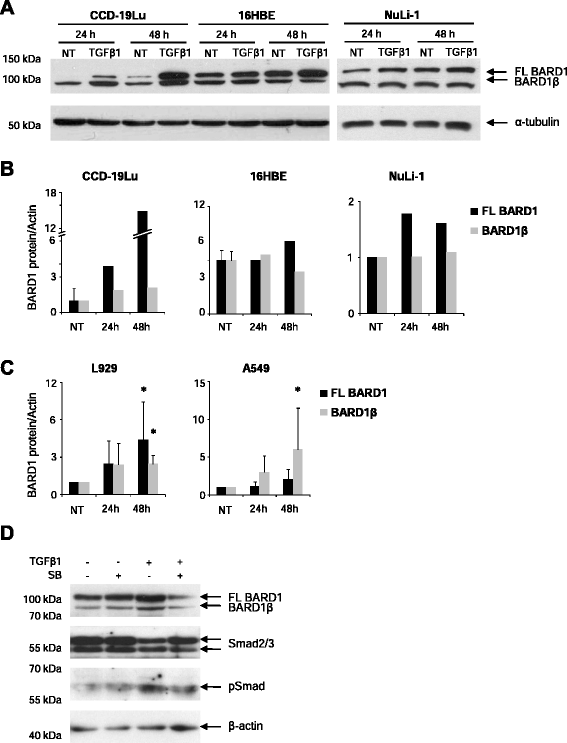
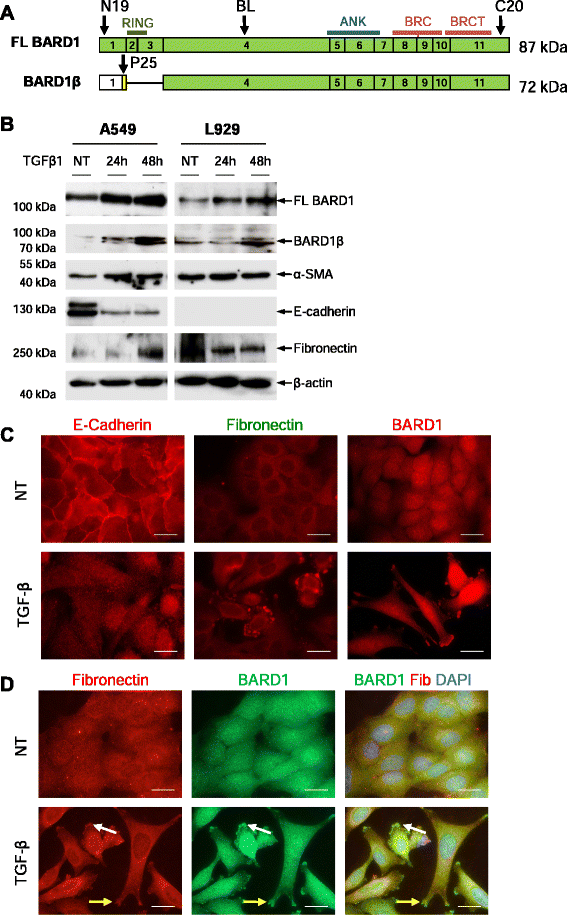
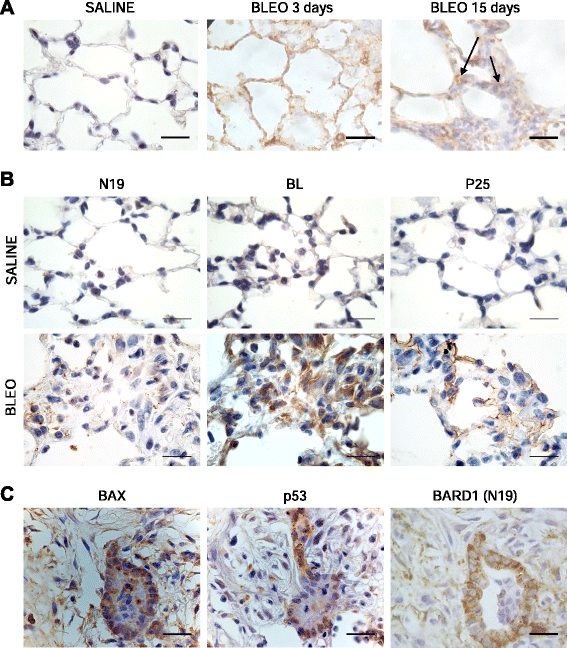
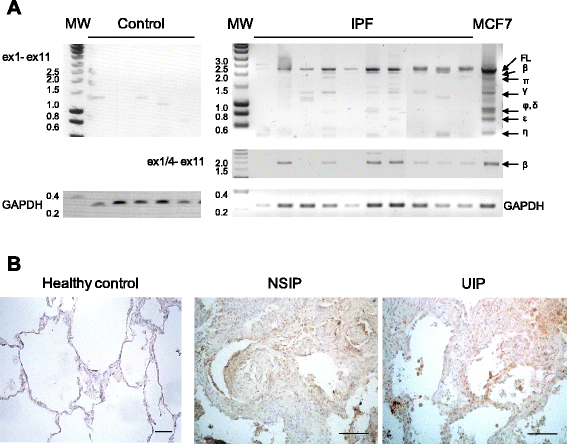
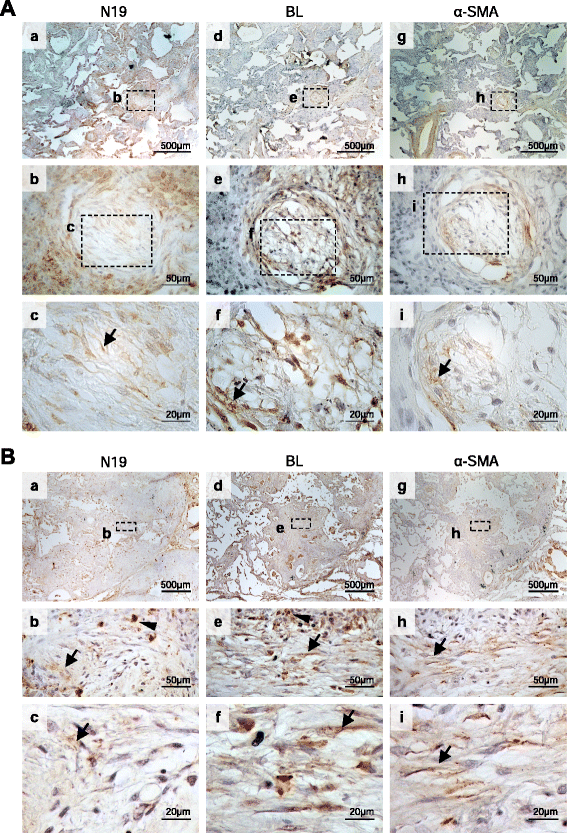
Results: FL BARD1 and BARD1β were upregulated in response to TGF-β in epithelial cells and fibroblasts in vitro and in vivo. Protein and mRNA expression studies showed very low expression in healthy lung tissues, but upregulated expression of full length (FL) BARD1 and BARD1β in fibrotic tissues.
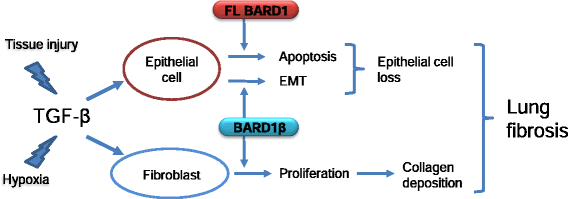
Conclusion: Our data suggest that FL BARD1 and BARD1β might be mediators of pleiotropic effects of TGF-β. In particular BARD1β might be a driver of proliferation and of pulmonary fibrosis pathogenesis and progression and represent a target for treatment.
Figures
References
-
- Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, et al. Transforming growth factors-beta 1, −beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997;150(3):981–991. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

