A chimeric prokaryotic pentameric ligand-gated channel reveals distinct pathways of activation
- PMID: 26415570
- PMCID: PMC4586589
- DOI: 10.1085/jgp.201511478
A chimeric prokaryotic pentameric ligand-gated channel reveals distinct pathways of activation
Abstract
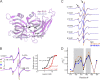
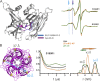
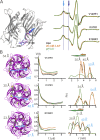
Recent high resolution structures of several pentameric ligand-gated ion channels have provided unprecedented details of their molecular architecture. However, the conformational dynamics and structural rearrangements that underlie gating and allosteric modulation remain poorly understood. We used a combination of electrophysiology, double electron-electron resonance (DEER) spectroscopy, and x-ray crystallography to investigate activation mechanisms in a novel functional chimera with the extracellular domain (ECD) of amine-gated Erwinia chrysanthemi ligand-gated ion channel, which is activated by primary amines, and the transmembrane domain of Gloeobacter violaceus ligand-gated ion channel, which is activated by protons. We found that the chimera was independently gated by primary amines and by protons. The crystal structure of the chimera in its resting state, at pH 7.0 and in the absence of primary amines, revealed a closed-pore conformation and an ECD that is twisted with respect to the transmembrane region. Amine- and pH-induced conformational changes measured by DEER spectroscopy showed that the chimera exhibits a dual mode of gating that preserves the distinct conformational changes of the parent channels. Collectively, our findings shed light on both conserved and divergent features of gating mechanisms in this class of channels, and will facilitate the design of better allosteric modulators.
© 2015 Schmandt et al.
Figures

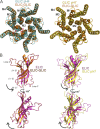

References
-
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W., et al. . 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

