A novel delivery platform based on Bacteriophage MS2 virus-like particles
- PMID: 26415756
- PMCID: PMC7114531
- DOI: 10.1016/j.virusres.2015.08.022
A novel delivery platform based on Bacteriophage MS2 virus-like particles
Abstract
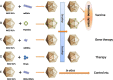
Our objective here is to review the novel delivery platform based on Bacteriophage MS2 virus-like particles (VLPs), including introduction to their structure, their potential as a delivery platform, and their expected use in medicine and other fields. Bacteriophage MS2 VLPs are nanoparticles devoid of viral genetic material and can self-assemble from the coat protein into an icosahedral capsid. As a novel delivery platform, they possess numerous features that make them suitable and attractive for targeted delivery of RNAs or DNAs, epitope peptides, and drugs within the protein capsid. In short, as a novel delivery platform, MS2 VLPs are suitable for delivery of targeted agents and hold promise for use in diagnostics, vaccines, and therapeutic modalities.
Keywords: Bacteriophage MS2; Delivery platform; In vitro diagnostic; Therapy; Vaccine; Virus-like particles.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

References
-
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989;337:709–716. - PubMed
-
- Ashley C.E., Carnes E.C., Phillips G.K., Durfee P.N., Buley M.D., Lino C.A., Padilla D.P., Phillips B., Carter M.B., Willman C.L., Brinker C.J., Caldeira J., do C., Chackerian B., Wharton W., Peabody D.S. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano. 2011;5:5729–5745. - PMC - PubMed
-
- Axblom C., Tars K., Fridborg K., Oma L., Bundule M., Liljas L. Structure of phage fr capsids with a deletion in the FG loop: implications for viral assembly. Virology. 1998;249:80–88. - PubMed
-
- Bachmann M.F., Rohrer U.H., Kündig T.M., Bürki K., Hengartner H., Zinkernagel R.M. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

