Inhibition of Alveolar Macrophage Pyroptosis Reduces Lipopolysaccharide-induced Acute Lung Injury in Mice
- PMID: 26415803
- PMCID: PMC4736856
- DOI: 10.4103/0366-6999.166039
Inhibition of Alveolar Macrophage Pyroptosis Reduces Lipopolysaccharide-induced Acute Lung Injury in Mice
Abstract
Background: Pyroptosis is the term for caspase-1-dependent cell death associated with pro-inflammatory cytokines. The role of alveolar macrophage (AM) pyroptosis in the pathogenesis of the acute lung injury and acute respiratory distress syndrome (ALI/ARDS) remains unclear.
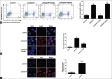
Methods: C57BL/6 wild-type mice were assigned to sham, lipopolysaccharide (LPS) + vehicle, LPS + acetyl-tyrosyl-valyl- alanyl-aspartyl-chloromethylketone (Ac-YVAD-CMK) and LPS + Z-Asp-Glu-Val-Asp-fluoromethylketone groups. Mice were given intraperitoneal (IP) injections of LPS. Drugs were IP injected 1 h before LPS administration. Mice were sacrificed 16 h after LPS administration, and AMs were isolated. Western blot analysis for active caspase-1 and cleaved caspase-3, evaluation of lung injury and a cytokine release analysis were performed. AMs were treated with LPS and adenosine triphosphate (ATP); caspase-1-dependent cell death was evaluated using flow cytometry; the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) pyroptosomes were examined by immunofluorescence.
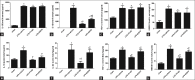
Results: The expression of activated caspase-1 in AMs was enhanced following LPS challenge compared with the sham group. In the ex vivo study, the caspase-1/propidium iodide-positive cells, caspase-1 specks and ASC pyroptosomes were up-regulated in AMs following LPS/ATP stimulation. The specific caspase-1 inhibitor Ac-YVAD-CMK inhibited the activation of caspase-1 and pyroptotic cell death. Ac-YVAD-CMK also reduced the lung injury, pulmonary edema and total protein in bronchoalveolar lavage fluid (BALF). In addition, Ac-YVAD-CMK significantly inhibited interleukin-α2 (IL-1α2) release both in serum and BALF and reduced the levels of IL-18, tumor necrosis factor-α± (TNF-α±), High Mobility Group Box 1 (HMGB1) in BALF during LPS-induced ALI/ARDS.
Conclusions: This study reported AM pyroptosis during LPS-induced ALI/ARDS in mice and has demonstrated that Ac-YVAD-CMK can prevent AM-induced pyroptosis and lung injury. These preliminary findings may form the basis for further studies to evaluate this pathway as a target for prevention or reduction of ALI/ARDS.
Figures


References
-
- Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. - PubMed
-
- Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, et al. The ALIEN study: Incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

