MiR-200b regulates autophagy associated with chemoresistance in human lung adenocarcinoma
- PMID: 26416454
- PMCID: PMC4741731
- DOI: 10.18632/oncotarget.5352
MiR-200b regulates autophagy associated with chemoresistance in human lung adenocarcinoma
Abstract
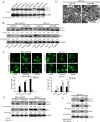
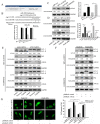
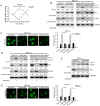
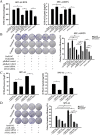
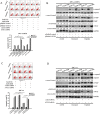
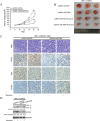
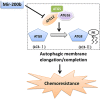
Chemoresistance remains a major clinical problem in combating human lung adenocarcinoma (LAD), and abnormal autophagy is closely associated with this phenomenon. In the present study, an inverse correlation between miR-200b and autophagy-associated gene 12 (ATG12) expressions was observed in docetaxel-resistant (SPC-A1/DTX and H1299/DTX) and sensitive (SPC-A1 and H1299) LAD cells as well as in tissue samples. Further study showed that miR-200b directly targeted ATG12 in LAD. Moreover, miR-200b-dependent ATG12 downregulation inhibited autophagy and enhanced the chemosensitivity of SPC-A1/DTX and H1299/DTX cells both in vivo and in vitro. LAD chemoresistance is therefore closely related to downregulation of miR-200b and the corresponding upregulation of ATG12. These results provide new evidence for the mechanisms governing the microRNA (miRNA)-ATG12 network and their possible contribution to autophagy modulation and LAD chemoresistance.
Keywords: ATG12; autophagy; chemoresistance; human lung adenocarcinoma; miR-200b.
Conflict of interest statement
The authors made no disclosures.
Figures

References
-
- Rosell R, Karachaliou N. Lung cancer in 2014. Optimizing lung cancer treatment approaches. Nature reviews Clinical oncology. 2015;12:75–76. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA: a cancer journal for clinicians. 2014;64:9–29. - PubMed
-
- Soejima K, Naoki K, Ishioka K, Nakamura M, Nakatani M, Kawada I, Watanabe H, Nakachi I, Yasuda H, Satomi R, Nakayama S, Yoda S, Terai H, et al. A phase II study of biweekly paclitaxel and carboplatin in elderly patients with advanced non-small cell lung cancer. Cancer chemotherapy and pharmacology. 2015;75:513–519. - PubMed
-
- Mizushima N. Autophagy: process and function. Genes & development. 2007;21:2861–2873. - PubMed
-
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiological reviews. 2010;90:1383–1435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

