Transcranial functional ultrasound imaging of the brain using microbubble-enhanced ultrasensitive Doppler
- PMID: 26416649
- PMCID: PMC4686564
- DOI: 10.1016/j.neuroimage.2015.09.037
Transcranial functional ultrasound imaging of the brain using microbubble-enhanced ultrasensitive Doppler
Abstract
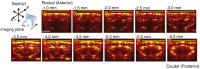
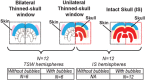
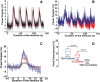
Functional ultrasound (fUS) is a novel neuroimaging technique, based on high-sensitivity ultrafast Doppler imaging of cerebral blood volume, capable of measuring brain activation and connectivity in rodents with high spatiotemporal resolution (100μm, 1ms). However, the skull attenuates acoustic waves, so fUS in rats currently requires craniotomy or a thinned-skull window. Here we propose a non-invasive approach by enhancing the fUS signal with a contrast agent, inert gas microbubbles. Plane-wave illumination of the brain at high frame rate (500Hz compounded sequence with three tilted plane waves, PRF=1500Hz with a 128 element 15MHz linear transducer), yields highly-resolved neurovascular maps. We compared fUS imaging performance through the intact skull bone (transcranial fUS) versus a thinned-skull window in the same animal. First, we show that the vascular network of the adult rat brain can be imaged transcranially only after a bolus intravenous injection of microbubbles, which leads to a 9dB gain in the contrast-to-tissue ratio. Next, we demonstrate that functional increase in the blood volume of the primary sensory cortex after targeted electrical-evoked stimulations of the sciatic nerve is observable transcranially in presence of contrast agents, with high reproducibility (Pearson's coefficient ρ=0.7±0.1, p=0.85). Our work demonstrates that the combination of ultrafast Doppler imaging and injection of contrast agent allows non-invasive functional brain imaging through the intact skull bone in rats. These results should ease non-invasive longitudinal studies in rodents and open a promising perspective for the adoption of highly resolved fUS approaches for the adult human brain.
Keywords: Blood volume; Functional ultrasound imaging; Microbubbles; Primary sensory cortex; Somatosensory activation; Transcranial.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bercoff J., Montaldo G., Loupas T., Savery D., Meziere F., Fink M., Tanter M. Ultrafast compound doppler imaging: providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011;58:134–147. - PubMed
-
- Burns P.N., Wilson S.R. Microbubble contrast for radiological imaging: 1. Principles. Ultrasound Q. 2006;22:5–13. - PubMed
-
- Cloutier G., Qin Z. Ultrasound backscattering from non-aggregating and aggregating erythrocytes--a review. Biorheology. 1997;34:443–470. - PubMed
-
- Couture O., Bannouf S., Montaldo G., Aubry J.-F., Fink M., Tanter M. Ultrafast imaging of ultrasound contrast agents. Ultrasound Med. Biol. 2009;35:1908–1916. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

