Determinant Factors of the Direct Medical Costs Associated with Genotype 1 Hepatitis C Infection in Treatment-Experienced Patients
- PMID: 26416653
- PMCID: PMC4662942
- DOI: 10.1007/s40268-015-0109-5
Determinant Factors of the Direct Medical Costs Associated with Genotype 1 Hepatitis C Infection in Treatment-Experienced Patients
Abstract
Objective: Limited evidence is available on predictors of medical resource utilization (MRU) and related direct costs, especially in treatment-experienced patients infected with genotype 1 hepatitis C virus (HCV). This study aimed at investigating patient and treatment characteristics that predict MRU and related non-drug costs in treatment-experienced patients with chronic hepatitis C (CHC) treated with simeprevir (SMV) or telapravir (TVR) in combination with pegylated interferon and ribavirin (PegIFN/R).
Patients and methods: A total of 709 patients who completed the 72-week ATTAIN trial were included in the study. Cost data were analysed from the UK NHS perspective. Descriptive statistics and regression analyses were used to determine patterns and predictors of total MRU-related costs associated with SMV/PegIFN/R and TVR/PegIFN/R.
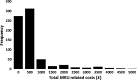
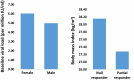
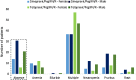
Results: Independent predictors for total MRU-related costs were age, region and the following interaction terms: (1) gender × F3-F4 METAVIR score × baseline viral load (BLVL), (2) body mass index (BMI) × F3-F4 METAVIR score × prior response to PegIFN/R and (3) gender × achievement of SVR at 12 weeks (SVR12) × BLVL. A F3-F4 METAVIR score was a stronger predictor of total MRU-related costs than SVR12. Predictors of adverse events included older age, female gender, low BMI, TVR/PegIFN/R and SVR12. Wilcoxon rank sum test revealed comparable total MRU-related costs between SMV/PegIFN/R and TVR/PegIFN/R.
Conclusion: To the best of our knowledge, this study is the first to describe the relationship between commonly admitted predictors of MRU-related costs and their joint effect on total MRU-related costs in treatment-experienced patients with CHC. The identified predictors of MRU-related costs suggest that significant treatment costs can be avoided by starting treatment early before the disease progresses. Furthermore, adverse events seem to be the most important factor to take into consideration for the choice of treatment, especially when therapeutic options are associated with similar levels of medical resource utilization and associated costs.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

