Cell-based therapy technology classifications and translational challenges
- PMID: 26416686
- PMCID: PMC4634004
- DOI: 10.1098/rstb.2015.0017
Cell-based therapy technology classifications and translational challenges
Abstract
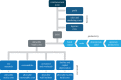
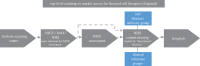
Cell therapies offer the promise of treating and altering the course of diseases which cannot be addressed adequately by existing pharmaceuticals. Cell therapies are a diverse group across cell types and therapeutic indications and have been an active area of research for many years but are now strongly emerging through translation and towards successful commercial development and patient access. In this article, we present a description of a classification of cell therapies on the basis of their underlying technologies rather than the more commonly used classification by cell type because the regulatory path and manufacturing solutions are often similar within a technology area due to the nature of the methods used. We analyse the progress of new cell therapies towards clinical translation, examine how they are addressing the clinical, regulatory, manufacturing and reimbursement requirements, describe some of the remaining challenges and provide perspectives on how the field may progress for the future.
Keywords: cell therapy; clinical trial; manufacturing; regulation; reimbursement; translation.
© 2015 The Authors.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
