The genomic landscape of response to EGFR blockade in colorectal cancer
- PMID: 26416732
- PMCID: PMC4878148
- DOI: 10.1038/nature14969
The genomic landscape of response to EGFR blockade in colorectal cancer
Abstract
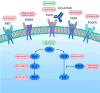
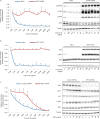
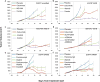
Colorectal cancer is the third most common cancer worldwide, with 1.2 million patients diagnosed annually. In late-stage colorectal cancer, the most commonly used targeted therapies are the monoclonal antibodies cetuximab and panitumumab, which prevent epidermal growth factor receptor (EGFR) activation. Recent studies have identified alterations in KRAS and other genes as likely mechanisms of primary and secondary resistance to anti-EGFR antibody therapy. Despite these efforts, additional mechanisms of resistance to EGFR blockade are thought to be present in colorectal cancer and little is known about determinants of sensitivity to this therapy. To examine the effect of somatic genetic changes in colorectal cancer on response to anti-EGFR antibody therapy, here we perform complete exome sequence and copy number analyses of 129 patient-derived tumour grafts and targeted genomic analyses of 55 patient tumours, all of which were KRAS wild-type. We analysed the response of tumours to anti-EGFR antibody blockade in tumour graft models and in clinical settings and functionally linked therapeutic responses to mutational data. In addition to previously identified genes, we detected mutations in ERBB2, EGFR, FGFR1, PDGFRA, and MAP2K1 as potential mechanisms of primary resistance to this therapy. Novel alterations in the ectodomain of EGFR were identified in patients with acquired resistance to EGFR blockade. Amplifications and sequence changes in the tyrosine kinase receptor adaptor gene IRS2 were identified in tumours with increased sensitivity to anti-EGFR therapy. Therapeutic resistance to EGFR blockade could be overcome in tumour graft models through combinatorial therapies targeting actionable genes. These analyses provide a systematic approach to evaluating response to targeted therapies in human cancer, highlight new mechanisms of responsiveness to anti-EGFR therapies, and delineate new avenues for intervention in managing colorectal cancer.
Conflict of interest statement
Competing financial interests: L.A.D. and V.E.V. are co-founders of Personal Genome Diagnostics and are members of its Scientific Advisory Board and Board of Directors. V.E.V. and L.A.D. own Personal Genome Diagnostics stock, which is subject to certain restrictions under University policy. The authors are entitled to a share of the royalties received by the University on sales of products related to genes described in this manuscript. The terms of these arrangements are managed by the Johns Hopkins University in accordance with its conflict-of-interest policies.
Figures

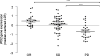









References
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, on behalf of the, E. G. W. G Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2014 doi: 10.1093/annonc/mdu260. - DOI - PubMed
-
- Amado RG, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. - PubMed
-
- De Roock W, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. The lancet oncology. 2010;11(10):753–762. 70130–3. doi: 10.1016/S1470-2045. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

