Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells
- PMID: 26416748
- PMCID: PMC4648562
- DOI: 10.1038/nature15260
Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells
Abstract
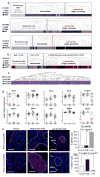
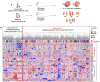
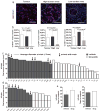
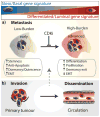
Despite major advances in understanding the molecular and genetic basis of cancer, metastasis remains the cause of >90% of cancer-related mortality. Understanding metastasis initiation and progression is critical to developing new therapeutic strategies to treat and prevent metastatic disease. Prevailing theories hypothesize that metastases are seeded by rare tumour cells with unique properties, which may function like stem cells in their ability to initiate and propagate metastatic tumours. However, the identity of metastasis-initiating cells in human breast cancer remains elusive, and whether metastases are hierarchically organized is unknown. Here we show at the single-cell level that early stage metastatic cells possess a distinct stem-like gene expression signature. To identify and isolate metastatic cells from patient-derived xenograft models of human breast cancer, we developed a highly sensitive fluorescence-activated cell sorting (FACS)-based assay, which allowed us to enumerate metastatic cells in mouse peripheral tissues. We compared gene signatures in metastatic cells from tissues with low versus high metastatic burden. Metastatic cells from low-burden tissues were distinct owing to their increased expression of stem cell, epithelial-to-mesenchymal transition, pro-survival, and dormancy-associated genes. By contrast, metastatic cells from high-burden tissues were similar to primary tumour cells, which were more heterogeneous and expressed higher levels of luminal differentiation genes. Transplantation of stem-like metastatic cells from low-burden tissues showed that they have considerable tumour-initiating capacity, and can differentiate to produce luminal-like cancer cells. Progression to high metastatic burden was associated with increased proliferation and MYC expression, which could be attenuated by treatment with cyclin-dependent kinase (CDK) inhibitors. These findings support a hierarchical model for metastasis, in which metastases are initiated by stem-like cells that proliferate and differentiate to produce advanced metastatic disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
-
- Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nature Rev Cancer. 2005;5:591–602. - PubMed
-
- Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. - PubMed
-
- Pang R, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. - PubMed
-
- Dieter SM, et al. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell. 2011;9:357–365. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

