Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014
- PMID: 26417060
- PMCID: PMC4677274
- DOI: 10.1093/eurheartj/ehv511
Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014
Abstract
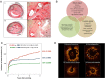
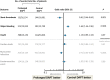
Modern-day stenting procedures leverage advances in pharmacotherapy and device innovation. Patients treated with contemporary antiplatelet agents, peri-procedural antithrombin therapy and new-generation drug-eluting stents (DES) have excellent outcomes over the short to medium term. Indeed, coupled with the reducing costs of these devices in most countries there remain very few indications where patients should be denied treatment with standard-of-care DES therapy. The two major causes of stent failure are stent thrombosis (ST) and in-stent restenosis (ISR). The incidence of both has reduced considerably in recent years. Current clinical registries and randomized trials with broad inclusion criteria show rates of ST at or <1% after 1 year and ∼0.2-0.4% per year thereafter; rates of clinical ISR are 5% respectively. Angiographic surveillance studies in large cohorts show rates of angiographic ISR of ∼10% with new-generation DES. The advent of high-resolution intracoronary imaging has shown that in many cases of late stent failure neoatherosclerotic change within the stented segment represents a final common pathway for both thrombotic and restenotic events. In future, a better understanding of the pathogenesis of this process may translate into improved late outcomes. Moreover, the predominance of non-stent-related disease as a cause of subsequent myocardial infarction during follow-up highlights the importance of lifestyle and pharmacological interventions targeted at modification of the underlying disease process. Finally, although recent developments focus on strategies which circumvent the need for chronically indwelling stents--such as drug-coated balloons or fully bioresorbable stents-more data are needed before the wider use of these therapies can be advocated.
Keywords: Bioresorbable stents; Coronary artery disease; Drug-eluting stents; In-stent restenosis; Neoatherosclerosis; Stent thrombosis.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

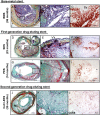
References
-
- Grüntzig A. Transluminal dilatation of coronary artery stenosis. The Lancet 1978;311:263-263. - PubMed
-
- Meier B. The first patient to undergo coronary angioplasty – 23-year follow-up. N Engl J Med 2001;344:144–145. - PubMed
-
- Monagan D. Journey into the Heart. USA: Gotham Books and Penguin; 2007.
-
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701–706. - PubMed
-
- Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, Belardi J, Sigwart U, Colombo A, Goy JJ, van den Heuvel P, Delcan J, Marie-angele Morel for the Benestent Study Group. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

