Endothelial miR-17∼92 cluster negatively regulates arteriogenesis via miRNA-19 repression of WNT signaling
- PMID: 26417068
- PMCID: PMC4611600
- DOI: 10.1073/pnas.1507094112
Endothelial miR-17∼92 cluster negatively regulates arteriogenesis via miRNA-19 repression of WNT signaling
Abstract
The contribution of endothelial-derived miR-17∼92 to ischemia-induced arteriogenesis has not been investigated in an in vivo model. In the present study, we demonstrate a critical role for the endothelial-derived miR-17∼92 cluster in shaping physiological and ischemia-triggered arteriogenesis. Endothelial-specific deletion of miR-17∼92 results in an increase in collateral density limbs and hearts and in ischemic limbs compared with control mice, and consequently improves blood flow recovery. Individual cluster components positively or negatively regulate endothelial cell (EC) functions in vitro, and, remarkably, ECs lacking the cluster spontaneously form cords in a manner rescued by miR-17a, -18a, and -19a. Using both in vitro and in vivo analyses, we identified FZD4 and LRP6 as targets of miR-19a/b. Both of these targets were up-regulated in 17∼92 KO ECs compared with control ECs, and both were shown to be targeted by miR-19 using luciferase assays. We demonstrate that miR-19a negatively regulates FZD4, its coreceptor LRP6, and WNT signaling, and that antagonism of miR-19a/b in aged mice improves blood flow recovery after ischemia and reduces repression of these targets. Collectively, these data provide insights into miRNA regulation of arterialization and highlight the importance of vascular WNT signaling in maintaining arterial blood flow.
Keywords: arteriogenesis; endothelium; microRNA; vascular.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
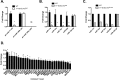





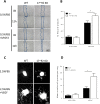
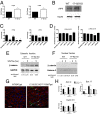


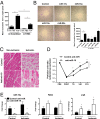


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL126933/HL/NHLBI NIH HHS/United States
- R01 HL61371/HL/NHLBI NIH HHS/United States
- R01HL126933/HL/NHLBI NIH HHS/United States
- R01 HL64793/HL/NHLBI NIH HHS/United States
- T32 GM007324/GM/NIGMS NIH HHS/United States
- R01 HL064793/HL/NHLBI NIH HHS/United States
- R56 HL117064/HL/NHLBI NIH HHS/United States
- R37 HL061371/HL/NHLBI NIH HHS/United States
- F32 HL107078/HL/NHLBI NIH HHS/United States
- R56HL117064/HL/NHLBI NIH HHS/United States
- R01 GM112182/GM/NIGMS NIH HHS/United States
- P01 HL1070295/HL/NHLBI NIH HHS/United States
- R01 HL081190/HL/NHLBI NIH HHS/United States
- R01 HL061371/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

