In vivo microscopy of hemozoin: towards a needle free diagnostic for malaria
- PMID: 26417515
- PMCID: PMC4574671
- DOI: 10.1364/BOE.6.003462
In vivo microscopy of hemozoin: towards a needle free diagnostic for malaria
Abstract
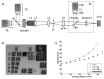
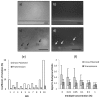
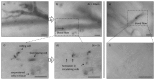
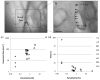
Clinical diagnosis of malaria suffers from poor specificity leading to overtreatment with antimalarial medications. Alternatives, like blood smear microscopy or antigen-based tests, require a blood sample. We investigate in vivo microscopy as a needle-free malaria diagnostic. Two optical signatures, birefringence and absorbance, of the endogenous malaria by-product hemozoin were evaluated as in vivo optical biomarkers. Hemozoin birefringence was difficult to detect in highly scattering tissue; however, hemozoin absorbance was observed in increasingly complex biological environments and detectable over a clinically-relevant range of parasitemia in vivo in a P. yoelii-infected mouse model of malaria.
Keywords: (170.0180) Microscopy; (170.1470) Blood or tissue constituent monitoring; (260.1440) Birefringence.
Figures


References
-
- World Health Organization, World Malaria Report (WHO Press, 2012).
LinkOut - more resources
Full Text Sources
