The anterior paraventricular thalamus modulates neuronal excitability in the suprachiasmatic nuclei of the rat
- PMID: 26417679
- PMCID: PMC4737286
- DOI: 10.1111/ejn.13088
The anterior paraventricular thalamus modulates neuronal excitability in the suprachiasmatic nuclei of the rat
Abstract
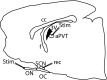
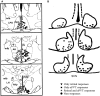
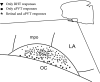
The suprachiasmatic nucleus (SCN) in mammals is the master clock which regulates circadian rhythms. Neural activity of SCN neurons is synchronized to external light through the retinohypothalamic tract (RHT). The paraventricular thalamic nucleus (PVT) is a neural structure that receives synaptic inputs from, and projects back to, the SCN. Lesioning the anterior PVT (aPVT) modifies the behavioral phase response curve induced by short pulses of bright light. In order to study the influence of the aPVT on SCN neural activity, we addressed whether the stimulation of the aPVT can modulate the electrical response of the SCN to either retinal or RHT stimulation. Using in vitro and in vivo recordings, we found a large population of SCN neurons responsive to the stimulation of either aPVT or RHT pathways. Furthermore, we found that simultaneous stimulation of the aPVT and the RHT increased neuronal responsiveness and spontaneous firing rate (SFR) in neurons with a low basal SFR (which also have more negative membrane potentials), such as quiescent and arrhythmic neurons, but no change was observed in neurons with rhythmic firing patterns and more depolarized membrane potentials. These results suggest that inputs from the aPVT could shift the membrane potential of an SCN neuron to values closer to its firing threshold and thus contribute to integration of the response of the circadian clock to light.
Keywords: circadian; hypothalamus; intralaminar nuclei; light entrainment; patch-clamp.
© 2015 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures






References
-
- Alamilla, J. & Aguilar‐Roblero, R. (2010) Glutamate and GABA neurotransmission from the paraventricular thalamus to the suprachiasmatic nuclei in the rat. J. Biol. Rhythm, 25, 28–36. - PubMed
-
- Belle, M.D. , Diekman, C.O. , Forger, D.B. & Piggins, H.D. (2009) Daily electrical silencing in the mammalian circadian clock. Science, 326, 281–284. - PubMed
-
- Bos, N.P. & Mirmiran, M. (1993) Effects of excitatory and inhibitory amino acids on neuronal discharges in the cultured suprachiasmatic nucleus. Brain Res. Bull., 31, 67–72. - PubMed
-
- Cavalcante, J.S. , Costa, M.S. , Santee, U.R. & Britto, L.R. (2005) Retinal projections to the midline and intralaminar thalamic nuclei in the common marmoset (Callithrix jacchus). Brain Res., 1043, 42–47. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

