Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing
- PMID: 26417955
- PMCID: PMC4785051
- DOI: 10.1002/cpt.269
Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing
Abstract
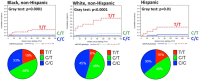
The antiretroviral protease inhibitor atazanavir inhibits hepatic uridine diphosphate glucuronosyltransferase (UGT) 1A1, thereby preventing the glucuronidation and elimination of bilirubin. Resultant indirect hyperbilirubinemia with jaundice can cause premature discontinuation of atazanavir. Risk for bilirubin-related discontinuation is highest among individuals who carry two UGT1A1 decreased function alleles (UGT1A1*28 or *37). We summarize published literature that supports this association and provide recommendations for atazanavir prescribing when UGT1A1 genotype is known (updates at www.pharmgkb.org).
© 2015 American Society for Clinical Pharmacology and Therapeutics.
Figures
References
-
- Court, M.H. et al Quantitative distribution of mRNAs encoding the 19 human UDP‐glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica 42, 266–277 (2012). - PubMed
-
- Bosma, P.J. et al Bilirubin UDP‐glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem. 269, 17960–17964 (1994). - PubMed
-
- Strassburg, C.P. Pharmacogenetics of Gilbert's syndrome. Pharmacogenomics 9, 703–715 (2008). - PubMed
-
- Kadakol, A. et al Genetic lesions of bilirubin uridine‐diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler‐Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum. Mutat. 16, 297–306 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL0105198/HL/NHLBI NIH HHS/United States
- U01 HL105198/HL/NHLBI NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- R24 GM061374/GM/NIGMS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R24 GM115264/GM/NIGMS NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- U01 GM061393/GM/NIGMS NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UO1 GM92666/GM/NIGMS NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- TR000445/TR/NCATS NIH HHS/United States
- R24 GM61374/GM/NIGMS NIH HHS/United States
- U01 GM092666/GM/NIGMS NIH HHS/United States
- U01 AI046339/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
- R01 GM102130/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

