Visualization of Active Glucocerebrosidase in Rodent Brain with High Spatial Resolution following In Situ Labeling with Fluorescent Activity Based Probes
- PMID: 26418157
- PMCID: PMC4587854
- DOI: 10.1371/journal.pone.0138107
Visualization of Active Glucocerebrosidase in Rodent Brain with High Spatial Resolution following In Situ Labeling with Fluorescent Activity Based Probes
Abstract
Gaucher disease is characterized by lysosomal accumulation of glucosylceramide due to deficient activity of lysosomal glucocerebrosidase (GBA). In cells, glucosylceramide is also degraded outside lysosomes by the enzyme glucosylceramidase 2 (GBA2) of which inherited deficiency is associated with ataxias. The interest in GBA and glucosylceramide metabolism in the brain has grown following the notion that mutations in the GBA gene impose a risk factor for motor disorders such as α-synucleinopathies. We earlier developed a β-glucopyranosyl-configured cyclophellitol-epoxide type activity based probe (ABP) allowing in vivo and in vitro visualization of active molecules of GBA with high spatial resolution. Labeling occurs through covalent linkage of the ABP to the catalytic nucleophile residue in the enzyme pocket. Here, we describe a method to visualize active GBA molecules in rat brain slices using in vivo labeling. Brain areas related to motor control, like the basal ganglia and motor related structures in the brainstem, show a high content of active GBA. We also developed a β-glucopyranosyl cyclophellitol-aziridine ABP allowing in situ labeling of GBA2. Labeled GBA2 in brain areas can be identified and quantified upon gel electrophoresis. The distribution of active GBA2 markedly differs from that of GBA, being highest in the cerebellar cortex. The histological findings with ABP labeling were confirmed by biochemical analysis of isolated brain areas. In conclusion, ABPs offer sensitive tools to visualize active GBA and to study the distribution of GBA2 in the brain and thus may find application to establish the role of these enzymes in neurodegenerative disease conditions such as α-synucleinopathies and cerebellar ataxia.
Conflict of interest statement
Figures
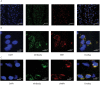



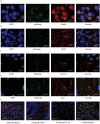

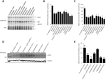

References
-
- Beutler E, Grabowski GA. Gaucher disease, The Metabolic and Molecular Bases of Inherited Disease (8th ed.). New York: Scriver CR, Sly WS, Valle D. (Eds.), McGraw-Hill, 2001; 3635–3668.
-
- van Weely S, Brandsma M, Strijland A, Tager JM, Aerts JM. Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochim Biophys Acta 1993; 1181:55–62. - PubMed
-
- Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 2004; 82:192–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

