Layer-by-Layers of Polymeric Micelles as a Biomimetic Drug-Releasing Network
- PMID: 26418812
- PMCID: PMC4752428
- DOI: 10.1002/mabi.201500310
Layer-by-Layers of Polymeric Micelles as a Biomimetic Drug-Releasing Network
Abstract
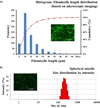
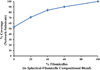
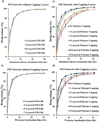
Mucin networks are lubricous biofunctional coats formed through the continuous deposition of mucin glycoproteins. Previously, we demonstrated the synthesis of a mucin mimic using biotinylated-filomicelles crosslinked via streptavidin using a layer-by-layer approach. These networks recreate the fibrous nature of mucin and can serve as a drug-releasing network. In this work, the ability to vary the network properties by blending filomicelles with spherical micelles is demonstrated. In addition, the deposition of a dense polymer coating on the mucin network was shown to act as a barrier to control diffusion and improved the structural stability under simulated oral chemical conditions. These biomimetic coatings can be utilized as a delivery system, providing a tunable drug release for oral applications.
Keywords: biomimetic; biotin-streptavidin; filomicelles; mucin; oral drug delivery.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





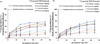


References
-
- Rogers DF. The European respiratory journal. 1994;7:1690. - PubMed
-
- Tabak LA, Levine MJ, Mandel ID, Ellison SA. J Oral Pathol Med. 1982;11:1. - PubMed
-
- Kim Y, Dalhaimer P, Christian DA, Discher DE. Nanotechnology. 2005;16:S484. - PubMed
-
- Prakobphol A, Levine MJ, Tabak LA, Reddy MS. Carbohydrate research. 1982;108:111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

