BPTF promotes tumor growth and predicts poor prognosis in lung adenocarcinomas
- PMID: 26418899
- PMCID: PMC4741809
- DOI: 10.18632/oncotarget.5302
BPTF promotes tumor growth and predicts poor prognosis in lung adenocarcinomas
Abstract
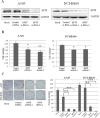
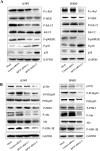
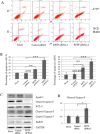
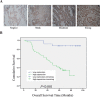
BPTF, a subunit of NURF, is well known to be involved in the development of eukaryotic cell, but little is known about its roles in cancers, especially in non-small-cell lung cancer (NSCLC). Here we showed that BPTF was specifically overexpressed in NSCLC cell lines and lung adenocarcinoma tissues. Knockdown of BPTF by siRNA significantly inhibited cell proliferation, induced cell apoptosis and arrested cell cycle progress from G1 to S phase. We also found that BPTF knockdown downregulated the expression of the phosphorylated Erk1/2, PI3K and Akt proteins and induced the cleavage of caspase-8, caspase-7 and PARP proteins, thereby inhibiting the MAPK and PI3K/AKT signaling and activating apoptotic pathway. BPTF knockdown by siRNA also upregulated the cell cycle inhibitors such as p21 and p18 but inhibited the expression of cyclin D, phospho-Rb and phospho-cdc2 in lung cancer cells. Moreover, BPTF knockdown by its specific shRNA inhibited lung cancer growth in vivo in the xenografts of A549 cells accompanied by the suppression of VEGF, p-Erk and p-Akt expression. Immunohistochemical assay for tumor tissue microarrays of lung tumor tissues showed that BPTF overexpression predicted a poor prognosis in the patients with lung adenocarcinomas. Therefore, our data indicate that BPTF plays an essential role in cell growth and survival by targeting multiply signaling pathways in human lung cancers.
Keywords: BPTF; lung cancer; prognosis; tumor growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA: a cancer journal for clinicians. 2012:2015. - PubMed
-
- Grose D, Devereux G, Milroy R. Comorbidity in lung cancer: important but neglected. a review of the current literature. Clinical lung cancer. 2011;12:207–211. - PubMed
-
- Ryan RJ, Bernstein BE. Molecular biology. Genetic events that shape the cancer epigenome. Science. 2012;336:1513–1514. - PubMed
-
- Neely KE, Workman JL. The complexity of chromatin remodeling and its links to cancer. Biochimica et biophysica acta. 2002;1603:19–29. - PubMed
-
- Bruce Alberts AJ, Julian Lewis, Martin Raff, Keith Roberts, Peter Walter. Molecular Biology of the Cell. 4. New York: Garland Science; 2002.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

