Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs)
- PMID: 26419531
- PMCID: PMC4588258
- DOI: 10.1186/s40168-015-0106-5
Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs)
Abstract
Background: Continuous-flow culture models are one tool for studying complex interactions between members of human fecal microbiotas because they allow studies to be completed during an extended period of time under conditions where pH, nutrient availability, and washout of waste products and dead cells can be controlled. Because many of the existing well-validated continuous-flow models are large and complex, we were interested in developing a simpler continuous-flow system that would allow microbial community dynamics to be examined in higher throughput while still maintaining complex microbial communities. To this end, we developed minibioreactor arrays (MBRAs), small volume bioreactors (15 ml) that allow simultaneous cultivation of up to 48 microbial communities in a single anaerobic chamber.
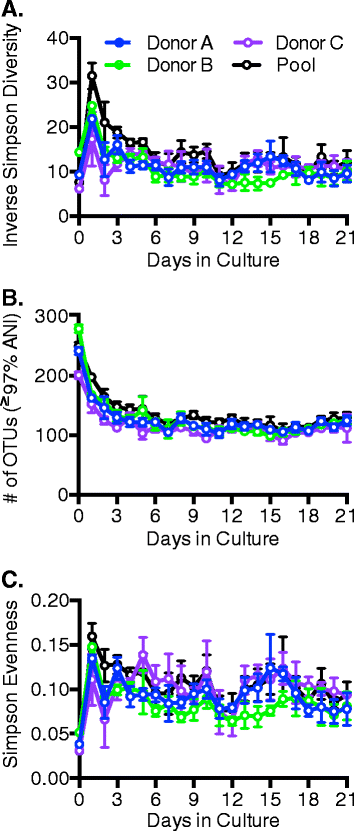
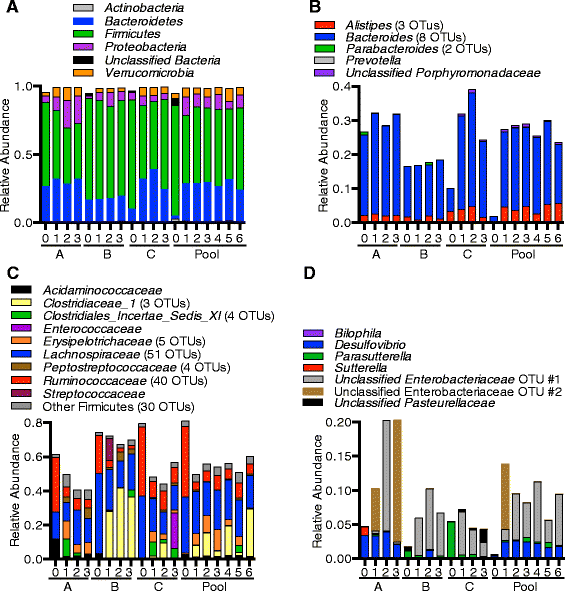
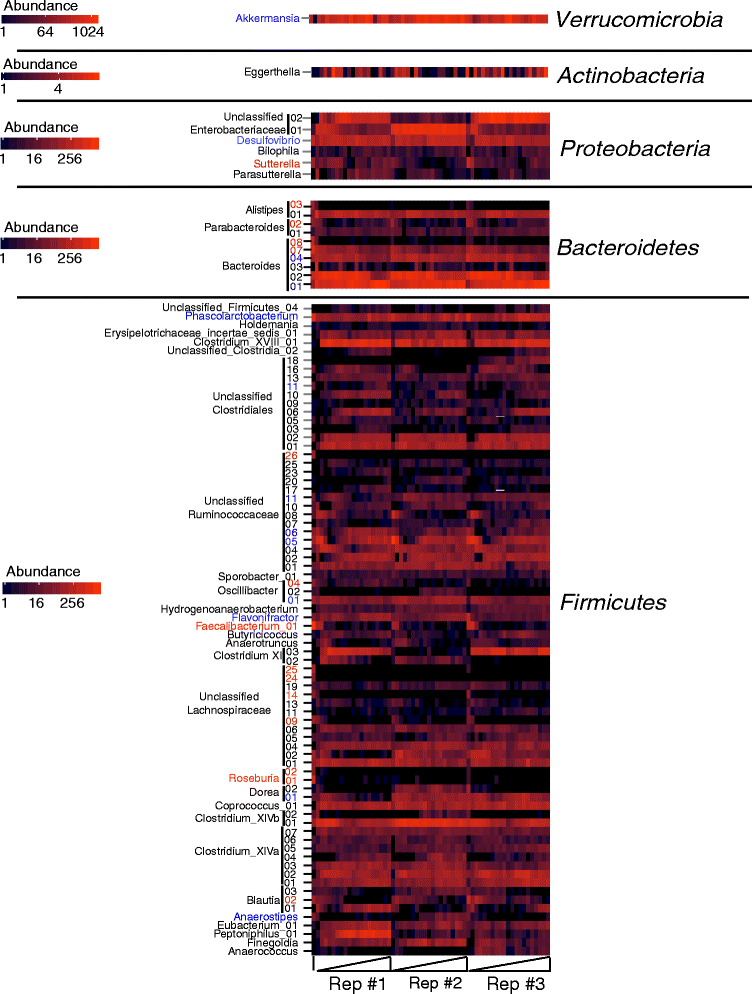
Results: We used MBRA to characterize the microbial community dynamics of replicate reactors inoculated from three different human fecal donors and reactors seeded with feces pooled from these three donors. We found that MBRA could be used to efficiently cultivate complex microbial communities that were a subset of the initial fecal inoculum (15-25 % of fecal OTUs initially observed). After an initial acclimation period of approximately 1 week, communities in each reactor stabilized and exhibited day-to-day variation similar to that observed in stable mouse fecal communities. Replicate reactors were predominately populated by shared core microbial communities; variation between replicate reactors was primarily driven by shifts in abundance of shared operational taxonomic units (OTUs). Consistent with differences between fecal donors, MBRA communities present in reactors seeded with different fecal samples had distinct composition and structure.
Conclusions: From these analyses, we conclude that MBRAs can be used to cultivate communities that recapitulate key features of human fecal communities and are a useful tool to facilitate higher-throughput studies of the dynamics of these communities.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

