A tunable azine covalent organic framework platform for visible light-induced hydrogen generation
- PMID: 26419805
- PMCID: PMC4598847
- DOI: 10.1038/ncomms9508
A tunable azine covalent organic framework platform for visible light-induced hydrogen generation
Abstract
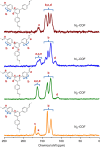
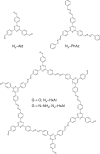
Hydrogen evolution from photocatalytic reduction of water holds promise as a sustainable source of carbon-free energy. Covalent organic frameworks (COFs) present an interesting new class of photoactive materials, which combine three key features relevant to the photocatalytic process, namely crystallinity, porosity and tunability. Here we synthesize a series of water- and photostable 2D azine-linked COFs from hydrazine and triphenylarene aldehydes with varying number of nitrogen atoms. The electronic and steric variations in the precursors are transferred to the resulting frameworks, thus leading to a progressively enhanced light-induced hydrogen evolution with increasing nitrogen content in the frameworks. Our results demonstrate that by the rational design of COFs on a molecular level, it is possible to precisely adjust their structural and optoelectronic properties, thus resulting in enhanced photocatalytic activities. This is expected to spur further interest in these photofunctional frameworks where rational supramolecular engineering may lead to new material applications.
Figures






References
-
- Ding S. Y. & Wang W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013). - PubMed
-
- Feng X., Ding X. S. & Jiang D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012). - PubMed
-
- Fang Q. et al.. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 5, 4503 (2014). - PubMed
-
- El-Kaderi H. M. et al.. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007). - PubMed
-
- Yu J.-T., Chen Z., Sun J., Huang Z.-T. & Zheng Q.-Y. Cyclotricatechylene based porous crystalline material: synthesis and applications in gas storage. J. Mater. Chem. 22, 5369–5373 (2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

