Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course
- PMID: 26419852
- PMCID: PMC4731438
- DOI: 10.1007/s00259-015-3193-4
Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course
Abstract
Purpose: In peptide receptor radionuclide therapy (PRRT), the bone marrow (BM) is one of the dose-limiting organs. The accepted dose limit for BM is 2 Gy, adopted from (131)I treatment. We investigated the incidence and duration of haematological toxicity and its risk factors in patients treated with PRRT with (177)Lu-DOTA(0)-Tyr(3)-octreotate ((177)Lu-DOTATATE). Also, absorbed BM dose estimates were evaluated and compared with the accepted 2 Gy dose limit.
Methods: The incidence and duration of grade 3 or 4 haematological toxicity (according to CTCAE v3.0) and risk factors were analysed. Mean BM dose per unit (gigabecquerels) of administered radioactivity was calculated and the correlations between doses to the BM and haematological risk factors were determined.
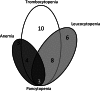
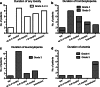
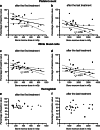
Results: Haematological toxicity (grade 3/4) occurred in 34 (11 %) of 320 patients. In 15 of the 34 patients, this lasted more than 6 months or blood transfusions were required. Risk factors significantly associated with haematological toxicity were: poor renal function, white blood cell (WBC) count <4.0 × 10(9)/l, age over 70 years, extensive tumour mass and high tumour uptake on the OctreoScan. Previous chemotherapy was not associated. The mean BM dose per administered activity in 23 evaluable patients was 67 ± 7 mGy/GBq, resulting in a mean BM dose of 2 Gy in patients who received four cycles of 7.4 GBq (177)Lu-DOTATATE. Significant correlations between (cumulative) BM dose and platelet and WBC counts were found in a selected group of patients.
Conclusion: The incidence of subacute haematological toxicity after PRRT with (177)Lu-DOTATATE is acceptable (11 %). Patients with impaired renal function, low WBC count, extensive tumour mass, high tumour uptake on the OctreoScan and/or advanced age are more likely to develop grade 3/4 haematological toxicity. The BM dose limit of 2 Gy, adopted from (131)I, seems not to be valid for PRRT with (177)Lu-DOTATATE.
Keywords: 177Lu-DOTATATE; Bone marrow; Dosimetry; PRRT; Toxicity.
Figures
References
-
- Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38(12):2125–35. - PubMed
-
- Swärd C, Bernhardt P, Ahlman H, Wängberg B, Forssell-Aronsson E, Larsson M, et al. [177Lu-DOTA 0-Tyr 3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: the value of measuring absorbed dose to the kidney. World J Surg. 2010;34(6):1368–1372. doi: 10.1007/s00268-009-0387-6. - DOI - PubMed
-
- Garkavij M, Nickel M, Sjögreen-Gleisner K, Ljungberg M, Ohlsson T, Wingårdh K, et al. 177Lu-[DOTA0, Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: analysis of dosimetry with impact on future therapeutic strategy. Cancer. 2010;116(4 Suppl):1084–1092. doi: 10.1002/cncr.24796. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

