Pre-differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke
- PMID: 26420220
- PMCID: PMC4588906
- DOI: 10.1186/s13287-015-0175-1
Pre-differentiation of human neural stem cells into GABAergic neurons prior to transplant results in greater repopulation of the damaged brain and accelerates functional recovery after transient ischemic stroke
Abstract
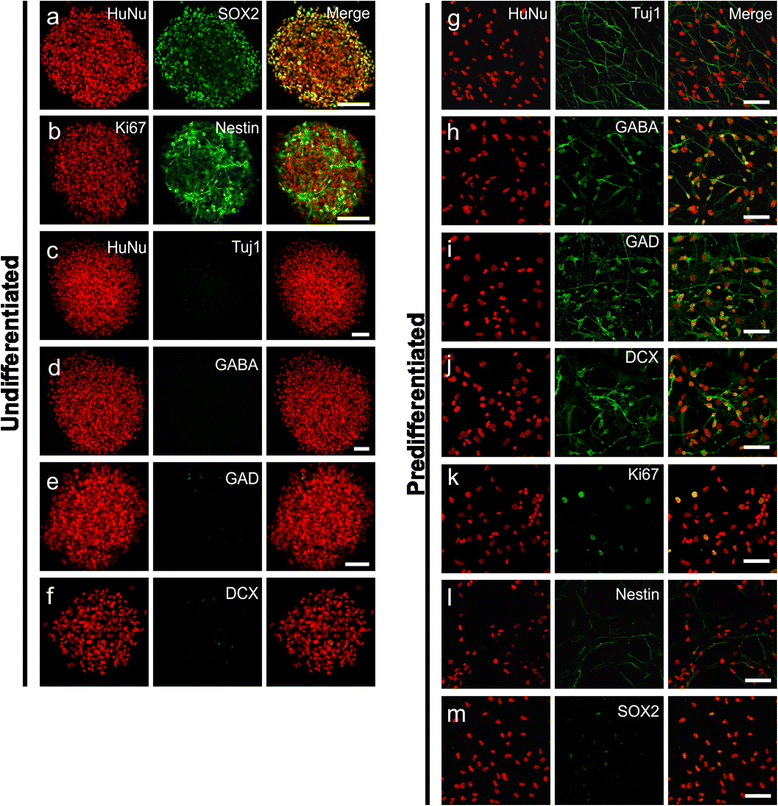
Introduction: Despite attempts to prevent brain injury during the hyperacute phase of stroke, most sufferers end up with significant neuronal loss and functional deficits. The use of cell-based therapies to recover the injured brain offers new hope. In the current study, we employed human neural stem cells (hNSCs) isolated from subventricular zone (SVZ), and directed their differentiation into GABAergic neurons followed by transplantation to ischemic brain.
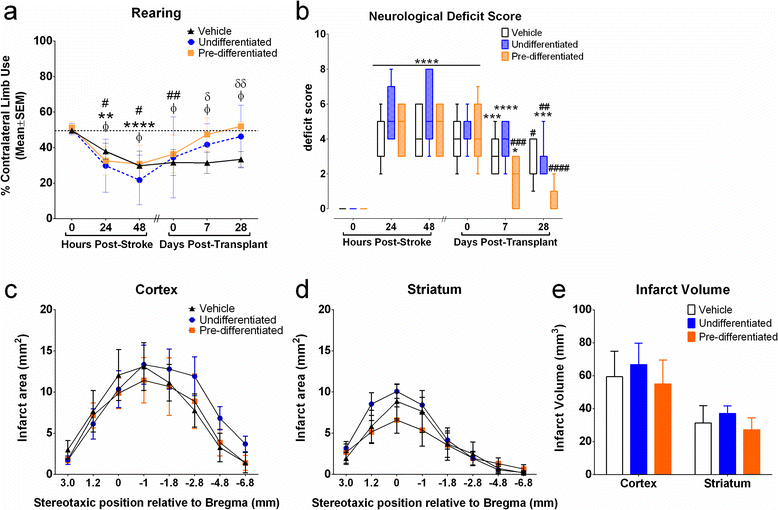
Methods: Pre-differentiated GABAergic neurons, undifferentiated SVZ-hNSCs or media alone were stereotaxically transplanted into the rat brain (n=7/group) 7 days after endothelin-1 induced stroke. Neurological outcome was assessed by neurological deficit scores and the cylinder test. Transplanted cell survival, cellular phenotype and maturation were assessed using immunohistochemistry and confocal microscopy.
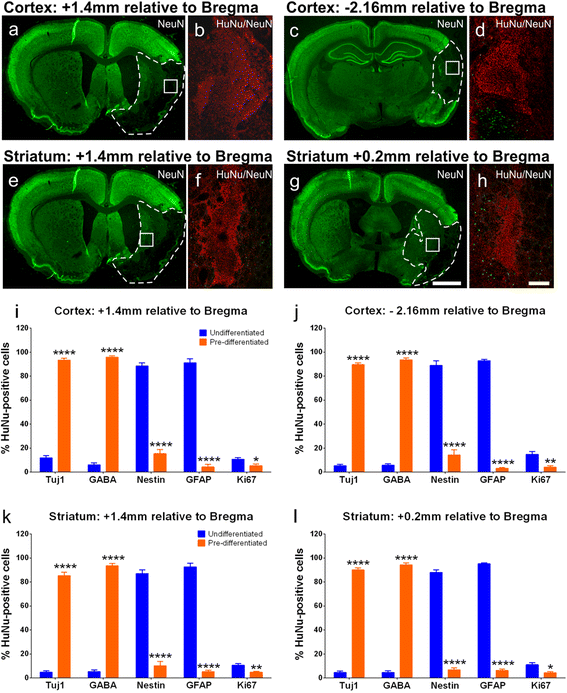
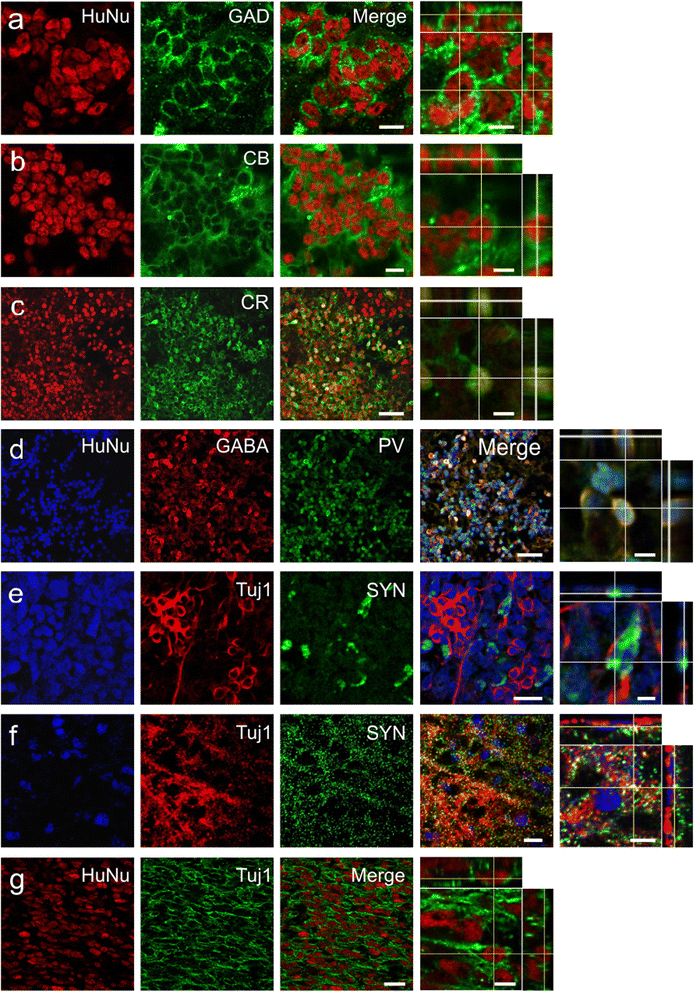
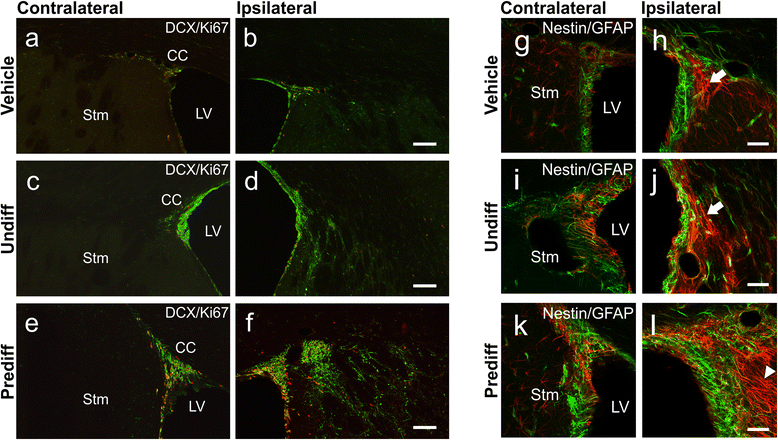
Results: Behavioral assessments revealed accelerated improvements in motor function 7 days post-transplant in rats treated with pre-differentiated GABAergic cells in comparison to media alone and undifferentiated hNSC treated groups. Histopathology 28 days-post transplant indicated that pre-differentiated cells maintained their GABAergic neuronal phenotype, showed evidence of synaptogenesis and up-regulated expression of both GABA and calcium signaling proteins associated with neurotransmission. Rats treated with pre-differentiated cells also showed increased neurogenic activity within the SVZ at 28 days, suggesting an additional trophic role of these GABAergic cells. In contrast, undifferentiated SVZ-hNSCs predominantly differentiated into GFAP-positive astrocytes and appeared to be incorporated into the glial scar.
Conclusion: Our study is the first to show enhanced exogenous repopulation of a neuronal phenotype after stroke using techniques aimed at GABAergic cell induction prior to delivery that resulted in accelerated and improved functional recovery.
Figures



References
-
- Nelles G. Cortical reorganization-effects of intensive therapy. Restor Neurol Neurosci. 2004;22:239–244. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

