Endoplasmic reticulum protein 29 (ERp29) confers radioresistance through the DNA repair gene, O(6)-methylguanine DNA-methyltransferase, in breast cancer cells
- PMID: 26420420
- PMCID: PMC4588584
- DOI: 10.1038/srep14723
Endoplasmic reticulum protein 29 (ERp29) confers radioresistance through the DNA repair gene, O(6)-methylguanine DNA-methyltransferase, in breast cancer cells
Abstract
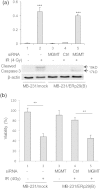
Resistance of cancer cells to radiotherapy is a major clinical problem in cancer treatment. Therefore, understanding the molecular basis of cellular resistance to radiotherapy and identification of novel targets are essential for improving treatment efficacy for cancer patients. Our previous studies have demonstrated a significant role of ERp29 in breast cancer cell survival against doxorubicin-induced genotoxic stress. We here reported that ERp29 expression in the triple negative MDA-MB-231 breast cancer cells significantly increased cell survival against ionizing radiation. Methylation PCR array analysis identified that ERp29 expression increased promoter hypomethylation of the DNA repair gene, O(6)-methylguanine DNA-methyltransferase (MGMT), by downregulating DNA methyltransferase 1. Knockdown of MGMT in the ERp29-transfected cancer cells increased radiosensitivity, leading to a decreased post-irradiation survival. In addition, radiation treatment in the MGMT-knockdown cells elevated phosphorylation of γ-H2AX and cleavage of caspase 3, indicating that depletion of MGMT facilitates DNA double strands breaks and increases cell apoptosis. Hence, our studies prove a novel function of ERp29\MGMT in cancer cell survival against radiation. Targeting ERp29\MGMT axis may be useful for providing better treatment efficacy in combination with radiotherapy in breast cancer.
Figures






References
-
- Zhang D. & Richardson D. R. Endoplasmic reticulum protein 29 (ERp29): An emerging role in cancer. Int J Biochem Cell Biol 43, 33–36 (2011). - PubMed
-
- Baryshev M., Sargsyan E. & Mkrtchian S. ERp29 is an essential endoplasmic reticulum factor regulating secretion of thyroglobulin. Biochemical and biophysical research communications 340, 617–624 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

