Ethosuximide Induces Hippocampal Neurogenesis and Reverses Cognitive Deficits in an Amyloid-β Toxin-induced Alzheimer Rat Model via the Phosphatidylinositol 3-Kinase (PI3K)/Akt/Wnt/β-Catenin Pathway
- PMID: 26420483
- PMCID: PMC4653709
- DOI: 10.1074/jbc.M115.652586
Ethosuximide Induces Hippocampal Neurogenesis and Reverses Cognitive Deficits in an Amyloid-β Toxin-induced Alzheimer Rat Model via the Phosphatidylinositol 3-Kinase (PI3K)/Akt/Wnt/β-Catenin Pathway
Abstract
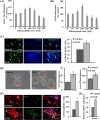
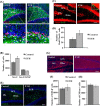
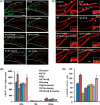
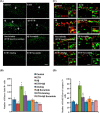
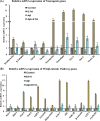
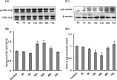
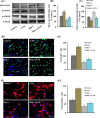
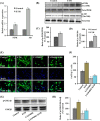
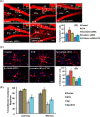
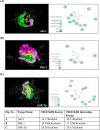
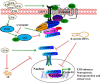
Neurogenesis involves generation of new neurons through finely tuned multistep processes, such as neural stem cell (NSC) proliferation, migration, differentiation, and integration into existing neuronal circuitry in the dentate gyrus of the hippocampus and subventricular zone. Adult hippocampal neurogenesis is involved in cognitive functions and altered in various neurodegenerative disorders, including Alzheimer disease (AD). Ethosuximide (ETH), an anticonvulsant drug is used for the treatment of epileptic seizures. However, the effects of ETH on adult hippocampal neurogenesis and the underlying cellular and molecular mechanism(s) are yet unexplored. Herein, we studied the effects of ETH on rat multipotent NSC proliferation and neuronal differentiation and adult hippocampal neurogenesis in an amyloid β (Aβ) toxin-induced rat model of AD-like phenotypes. ETH potently induced NSC proliferation and neuronal differentiation in the hippocampus-derived NSC in vitro. ETH enhanced NSC proliferation and neuronal differentiation and reduced Aβ toxin-mediated toxicity and neurodegeneration, leading to behavioral recovery in the rat AD model. ETH inhibited Aβ-mediated suppression of neurogenic and Akt/Wnt/β-catenin pathway gene expression in the hippocampus. ETH activated the PI3K·Akt and Wnt·β-catenin transduction pathways that are known to be involved in the regulation of neurogenesis. Inhibition of the PI3K·Akt and Wnt·β-catenin pathways effectively blocked the mitogenic and neurogenic effects of ETH. In silico molecular target prediction docking studies suggest that ETH interacts with Akt, Dkk-1, and GSK-3β. Our findings suggest that ETH stimulates NSC proliferation and differentiation in vitro and adult hippocampal neurogenesis via the PI3K·Akt and Wnt·β-catenin signaling.
Keywords: cell differentiation; cell proliferation; ethosuximide; hippocampus; neural stem cell (NSC); neurodegeneration; neurodegenerative disease; neurogenesis; neuron; neuroprotection.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Wennstrom M., Hellsten J., Ekstrand J., Lindgren H., and Tingstrom A. (2006) Corticosterone-induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol. Psychiatry 59, 178–186 - PubMed
-
- Zhao C., Deng W., and Gage F. H. (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 - PubMed
-
- Ming G. L., and Song H. (2005) Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

