Recent Advances in Discovering the Role of CCL5 in Metastatic Breast Cancer
- PMID: 26420723
- PMCID: PMC4968951
- DOI: 10.2174/138955751513150923094709
Recent Advances in Discovering the Role of CCL5 in Metastatic Breast Cancer
Abstract
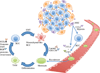
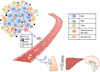
A variety of therapeutic strategies are currently under investigation to inhibit factors that promote tumor invasion, as metastasis is the most common cause of mortality for cancer patients. Notably, considerable emphasis has been placed on studying metastasis as a dynamic process that is highly dependent on the tumor microenvironment. In regards to breast cancer, chemokine C-C motif ligand 5 (CCL5), which is produced by tumor-associated stromal cells, has been established as an important contributor to metastatic disease. This review summarizes recent discoveries uncovering the role of this chemokine in breast cancer metastasis, including conditions that increase the generation of CCL5 and effects induced by this signaling pathway. In particular, CCL-5-mediated cancer cell migration and invasion are discussed in the context of intertwined feedback loops between breast cancer cells and stromal cells. Moreover, the potential use of CCL5 and its receptor chemokine C-C motif receptor 5 (CCR5) as targets for preventing breast cancer metastasis is also reviewed.
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Figures

Similar articles
-
The potential to target CCL5/CCR5 in breast cancer.Expert Opin Ther Targets. 2014 Nov;18(11):1265-75. doi: 10.1517/14728222.2014.949238. Epub 2014 Sep 26. Expert Opin Ther Targets. 2014. PMID: 25256399 Review.
-
Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases.Cell Adh Migr. 2013 May-Jun;7(3):315-24. doi: 10.4161/cam.25138. Epub 2013 May 24. Cell Adh Migr. 2013. PMID: 23722213 Free PMC article.
-
Endothelial cells promote triple-negative breast cancer cell metastasis via PAI-1 and CCL5 signaling.FASEB J. 2018 Jan;32(1):276-288. doi: 10.1096/fj.201700237RR. Epub 2017 Sep 12. FASEB J. 2018. PMID: 28899878
-
Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor‑associated macrophages.Oncol Rep. 2019 Dec;42(6):2499-2511. doi: 10.3892/or.2019.7344. Epub 2019 Oct 1. Oncol Rep. 2019. PMID: 31578575 Free PMC article.
-
Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment.Int J Mol Sci. 2022 Nov 16;23(22):14159. doi: 10.3390/ijms232214159. Int J Mol Sci. 2022. PMID: 36430633 Free PMC article. Review.
Cited by
-
Identification of differentially expressed lncRNAs and mRNAs in luminal-B breast cancer by RNA-sequencing.BMC Cancer. 2019 Dec 3;19(1):1171. doi: 10.1186/s12885-019-6395-5. BMC Cancer. 2019. PMID: 31795964 Free PMC article.
-
The Role and Therapeutic Targeting of CCR5 in Breast Cancer.Cells. 2023 Sep 8;12(18):2237. doi: 10.3390/cells12182237. Cells. 2023. PMID: 37759462 Free PMC article. Review.
-
2-Hydroxy-3-methylanthraquinone inhibits lung carcinoma cells through modulation of IL-6-induced JAK2/STAT3 pathway.Phytomedicine. 2019 Aug;61:152848. doi: 10.1016/j.phymed.2019.152848. Epub 2019 Jan 28. Phytomedicine. 2019. PMID: 31035048 Free PMC article.
-
Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention.CA Cancer J Clin. 2017 Sep;67(5):378-397. doi: 10.3322/caac.21405. Epub 2017 Aug 1. CA Cancer J Clin. 2017. PMID: 28763097 Free PMC article. Review.
-
Three-Dimensional Breast Cancer Model to Investigate CCL5/CCR1 Expression Mediated by Direct Contact between Breast Cancer Cells and Adipose-Derived Stromal Cells or Adipocytes.Cancers (Basel). 2023 Jul 5;15(13):3501. doi: 10.3390/cancers15133501. Cancers (Basel). 2023. PMID: 37444610 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
-
- Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5(8):591–602. - PubMed
-
- Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol. Med. Today. 2000;6(8):324–329. - PubMed
- Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–S72. - PubMed
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. - PubMed
- West RB, Nuyten DS, Subramanian S, Nielsen TO, Corless CL, Rubin BP, Montgomery K, Zhu S, Patel R, Hernandez-Boussard T, Goldblum JR, Brown PO, van de Vijver M, van de Rijn M. Determination of stromal signatures in breast carcinoma. PLoS Biol. 2005;3(6):e187. - PMC - PubMed
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008;66(1):1–9. - PubMed
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. - PubMed
-
- Bonafe M, Storci G, Franceschi C. Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example: breakdown of the multi-shell cytokine network fuels cancer in aged people. Bioessays. 2012;34(1):40–49. - PubMed
- Storci G, Bertoni S, De Carolis S, Papi A, Nati M, Ceccarelli C, Pirazzini C, Garagnani P, Ferrarini A, Buson G, Delledonne M, Fiorentino M, Capizzi E, Gruppioni E, Taffurelli M, Santini D, Franceschi C, Bandini G, Bonifazi F, Bonafe M. Slug/beta-catenin-dependent proinflammatory phenotype in hypoxic breast cancer stem cells. Am. J. Pathol. 2013;183(5):1688–1697. - PubMed
- An G, Kulkarni S. An agent-based modeling framework linking inflammation and cancer using evolutionary principles: Description of a generative hierarchy for the hallmarks of cancer and developing a bridge between mechanism and epidemiological data. Math. Biosci. 2014 - PMC - PubMed
- Segatto I, Berton S, Sonego M, Massarut S, Perin T, Piccoli E, Colombatti A, Vecchione A, Baldassarre G, Belletti B. Surgery-induced wound response promotes stem-like and tumor-initiating features of breast cancer cells, via STAT3 signaling. Oncotarget. 2014;5(15):6267–6279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

