Connectivity Map-based discovery of parbendazole reveals targetable human osteogenic pathway
- PMID: 26420877
- PMCID: PMC4611615
- DOI: 10.1073/pnas.1501597112
Connectivity Map-based discovery of parbendazole reveals targetable human osteogenic pathway
Abstract
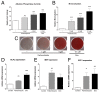
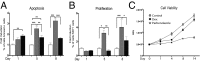
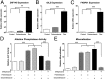
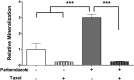
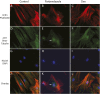
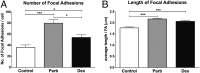
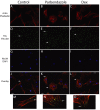
Osteoporosis is a common skeletal disorder characterized by low bone mass leading to increased bone fragility and fracture susceptibility. In this study, we have identified pathways that stimulate differentiation of bone forming osteoblasts from human mesenchymal stromal cells (hMSCs). Gene expression profiling was performed in hMSCs differentiated toward osteoblasts (at 6 h). Significantly regulated genes were analyzed in silico, and the Connectivity Map (CMap) was used to identify candidate bone stimulatory compounds. The signature of parbendazole matches the expression changes observed for osteogenic hMSCs. Parbendazole stimulates osteoblast differentiation as indicated by increased alkaline phosphatase activity, mineralization, and up-regulation of bone marker genes (alkaline phosphatase/ALPL, osteopontin/SPP1, and bone sialoprotein II/IBSP) in a subset of the hMSC population resistant to the apoptotic effects of parbendazole. These osteogenic effects are independent of glucocorticoids because parbendazole does not up-regulate glucocorticoid receptor (GR) target genes and is not inhibited by the GR antagonist mifepristone. Parbendazole causes profound cytoskeletal changes including degradation of microtubules and increased focal adhesions. Stabilization of microtubules by pretreatment with Taxol inhibits osteoblast differentiation. Parbendazole up-regulates bone morphogenetic protein 2 (BMP-2) gene expression and activity. Cotreatment with the BMP-2 antagonist DMH1 limits, but does not block, parbendazole-induced mineralization. Using the CMap we have identified a previously unidentified lineage-specific, bone anabolic compound, parbendazole, which induces osteogenic differentiation through a combination of cytoskeletal changes and increased BMP-2 activity.
Keywords: Connectivity Map; cytoskeleton; mesenchymal stem cell; osteoblast; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. - PubMed
-
- Kanis JA, Johnell O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int. 2005;16(3):229–238. - PubMed
-
- Lamb J, et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. - PubMed
-
- Qu XA, Rajpal DK. Applications of Connectivity Map in drug discovery and development. Drug Discov Today. 2012;17(23-24):1289–1298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

