A Comparison of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Insulin Injection in Children with Type I Diabetes in Kuwait: Glycemic Control, Insulin Requirement, and BMI
- PMID: 26421114
- PMCID: PMC4576387
- DOI: 10.5001/omj.2015.69
A Comparison of Continuous Subcutaneous Insulin Infusion vs. Multiple Daily Insulin Injection in Children with Type I Diabetes in Kuwait: Glycemic Control, Insulin Requirement, and BMI
Abstract
Objective: Continuous subcutaneous insulin infusion (CSII) and multiple daily insulin injections (MDI) are two methods currently used to manage type I diabetes mellitus (T1DM). Here we compare our experiences with CSII and MDI in a large cohort of pediatric patients in Kuwait.
Methods: Data on 326 patients with T1DM who were started on CSII between 2007 and 2012 were retrospectively compared with those of 326 patients on MDI. They were matched for sex, age at diagnosis, T1DM duration, glycemic control, insulin requirement, and body mass index (BMI). Data were collected at baseline and every three months and included glycated hemoglobin (HbA1c), insulin dose, and adverse events (severe hypoglycemia, diabetic ketoacidosis, and skin problems).
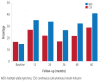
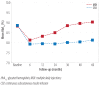
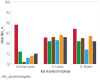
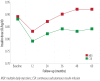
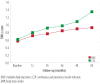
Results: The main reason for switching to CSII was to achieve better glycemic control (37%), followed by reducing hypoglycemia, and improving the quality of life (13.3% each). Although HbA1c decrease was most significant in the first year, it continued to be significantly lower in the CSII group compared to the MDI throughout the study period. Total daily insulin requirements were significantly lower in the CSII group. BMI increased in both groups, but the difference was significant only at the end of the fifth year. There was no significant change in the rate of diabetic ketoacidosis in either group. The CSII patients had more severe hypoglycemic episodes at baseline; however, it significantly decreased throughout the study period. Only five patients discontinued CSII therapy and two of these restarted within three months.
Conclusion: CSII is a safe intensive insulin therapy in youngsters with T1DM and achieved markedly fewer severe hypoglycemic episodes and lower daily insulin requirements.
Keywords: Adolescent; Body Mass Index; Child; Diabetes Mellitus, Type I; Hemoglobin A, Glycosylated; Insulin Infusion Systems.
Figures

References
-
- Diabetes Atlas-IDF. International Diabetes Federation, 4th Edition. 2013.
LinkOut - more resources
Full Text Sources
Other Literature Sources
