Patterns of Brain Activation and Meal Reduction Induced by Abdominal Surgery in Mice and Modulation by Rikkunshito
- PMID: 26421719
- PMCID: PMC4589401
- DOI: 10.1371/journal.pone.0139325
Patterns of Brain Activation and Meal Reduction Induced by Abdominal Surgery in Mice and Modulation by Rikkunshito
Abstract
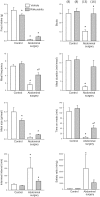
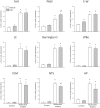
Abdominal surgery inhibits food intake and induces c-Fos expression in the hypothalamic and medullary nuclei in rats. Rikkunshito (RKT), a Kampo medicine improves anorexia. We assessed the alterations in meal microstructure and c-Fos expression in brain nuclei induced by abdominal surgery and the modulation by RKT in mice. RKT or vehicle was gavaged daily for 1 week. On day 8 mice had no access to food for 6-7 h and were treated twice with RKT or vehicle. Abdominal surgery (laparotomy-cecum palpation) was performed 1-2 h before the dark phase. The food intake and meal structures were monitored using an automated monitoring system for mice. Brain sections were processed for c-Fos immunoreactivity (ir) 2-h after abdominal surgery. Abdominal surgery significantly reduced bouts, meal frequency, size and duration, and time spent on meals, and increased inter-meal interval and satiety ratio resulting in 92-86% suppression of food intake at 2-24 h post-surgery compared with control group (no surgery). RKT significantly increased bouts, meal duration and the cumulative 12-h food intake by 11%. Abdominal surgery increased c-Fos in the prelimbic, cingulate and insular cortexes, and autonomic nuclei, such as the bed nucleus of the stria terminalis, central amygdala, hypothalamic supraoptic (SON), paraventricular and arcuate nuclei, Edinger-Westphal nucleus (E-W), lateral periaqueduct gray (PAG), lateral parabrachial nucleus, locus coeruleus, ventrolateral medulla and nucleus tractus solitarius (NTS). RKT induced a small increase in c-Fos-ir neurons in the SON and E-W of control mice, and in mice with surgery there was an increase in the lateral PAG and a decrease in the NTS. These findings indicate that abdominal surgery inhibits food intake by increasing both satiation (meal duration) and satiety (meal interval) and activates brain circuits involved in pain, feeding behavior and stress that may underlie the alterations of meal pattern and food intake inhibition. RKT improves food consumption post-surgically that may involve modulation of pain pathway.
Conflict of interest statement
Figures






Similar articles
-
Rikkunshito improves anorexia through ghrelin- and orexin-dependent activation of the brain hypothalamus and mesolimbic dopaminergic pathway in rats.Neurogastroenterol Motil. 2024 Nov;36(11):e14900. doi: 10.1111/nmo.14900. Epub 2024 Aug 20. Neurogastroenterol Motil. 2024. PMID: 39164871
-
Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats.Peptides. 2010 Feb;31(2):263-70. doi: 10.1016/j.peptides.2009.11.015. Epub 2009 Nov 26. Peptides. 2010. PMID: 19944727 Free PMC article.
-
Rikkunshito and 5-HT2C receptor antagonist improve cisplatin-induced anorexia via hypothalamic ghrelin interaction.Regul Pept. 2010 Apr 9;161(1-3):97-105. doi: 10.1016/j.regpep.2010.02.003. Epub 2010 Feb 19. Regul Pept. 2010. PMID: 20171995
-
Control of food intake by fatty acid oxidation and ketogenesis.Nutrition. 1999 Sep;15(9):704-14. doi: 10.1016/s0899-9007(99)00125-2. Nutrition. 1999. PMID: 10467616 Review.
-
Estradiol, CCK and satiation.Peptides. 2001 Aug;22(8):1251-63. doi: 10.1016/s0196-9781(01)00449-1. Peptides. 2001. PMID: 11457518 Review.
Cited by
-
Gut-Brain Neuroendocrine Signaling Under Conditions of Stress-Focus on Food Intake-Regulatory Mediators.Front Endocrinol (Lausanne). 2018 Aug 28;9:498. doi: 10.3389/fendo.2018.00498. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30210455 Free PMC article. Review.
-
Involvement of Ghrelin Dynamics in Stress-Induced Eating Disorder: Effects of Sex and Aging.Int J Mol Sci. 2021 Oct 28;22(21):11695. doi: 10.3390/ijms222111695. Int J Mol Sci. 2021. PMID: 34769125 Free PMC article. Review.
-
CRF receptor 1 antagonism and brain distribution of active components contribute to the ameliorative effect of rikkunshito on stress-induced anorexia.Sci Rep. 2016 Jun 8;6:27516. doi: 10.1038/srep27516. Sci Rep. 2016. PMID: 27273195 Free PMC article.
-
Effects of rikkunshito on renal fibrosis and inflammation in angiotensin II-infused mice.Sci Rep. 2019 Apr 17;9(1):6201. doi: 10.1038/s41598-019-42657-1. Sci Rep. 2019. PMID: 30996242 Free PMC article.
-
Regulation of the firing activity by PKA-PKC-Src family kinases in cultured neurons of hypothalamic arcuate nucleus.J Neurosci Res. 2020 Feb;98(2):384-403. doi: 10.1002/jnr.24516. Epub 2019 Aug 12. J Neurosci Res. 2020. PMID: 31407399 Free PMC article.
References
-
- Bonaz B, Tache Y. Corticotropin-releasing factor and systemic capsaicin-sensitive afferents are involved in abdominal surgery-induced Fos expression in the paraventricular nucleus of the hypothalamus. Brain Res 1997; 748(1–2):12–20. - PubMed
-
- Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Tache Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol 1996; 270(4 Pt 2):R888–R894. - PubMed
-
- Bonaz B, Plourde V, Tache Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J Comp Neurol 1994; 349(2):212–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

