Localization of CD8 T cell epitope within cardiac myosin heavy chain-α334-352 that induces autoimmune myocarditis in A/J mice
- PMID: 26422020
- PMCID: PMC4656055
- DOI: 10.1016/j.ijcard.2015.09.016
Localization of CD8 T cell epitope within cardiac myosin heavy chain-α334-352 that induces autoimmune myocarditis in A/J mice
Abstract
Background: Cardiac myosin heavy chain-α (Myhc), an intracellular protein expressed in the cardiomyocytes, has been identified as a major autoantigen in cardiac autoimmunity. In our studies with Myhc334-352-induced experimental autoimmune myocarditis in A/J mice (H-2a), we discovered that Myhc334-352, supposedly a CD4 T cell epitope, also induced antigen-specific CD8 T cells that transfer disease to naive animals.
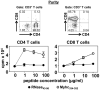
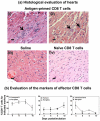
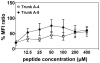
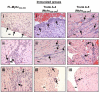
Methods and results: In our efforts to identify the CD8 T cell determinants, we localized Myhc338-348 within the full length-Myhc334-352, leading to four key findings. (1) By acting as a dual epitope, Myhc338-348 induces both CD4 and CD8 T cell responses. (2) In a major histocompatibility complex (MHC) class I-stabilization assay, Myhc338-348 was found to bind H-2Dd-but not H-2Kk or H-2Ld-alleles. (3) The CD8 T cell response induced by Myhc338-348 was antigen-specific, as evaluated by MHC class I/H-2Dd dextramer staining. The antigen-sensitized T cells predominantly produced interferon-γ, the critical cytokine of effector cytotoxic T lymphocytes. (4) Myhc338-348 was found to induce myocarditis in immunized animals as determined by histology and magnetic resonance microscopy imaging.
Conclusions: Our data provide new insights as to how different immune cells can recognize the same antigen and inflict damage through different mechanisms.
Keywords: Autoimmunity; CD8 T cells; Cardiac myosin; MHC class I dextramers; Myocarditis.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures




Similar articles
-
Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart.J Exp Med. 1995 Nov 1;182(5):1291-300. doi: 10.1084/jem.182.5.1291. J Exp Med. 1995. PMID: 7595200 Free PMC article.
-
Identification of novel mimicry epitopes for cardiac myosin heavy chain-α that induce autoimmune myocarditis in A/J mice.Cell Immunol. 2011;271(2):438-49. doi: 10.1016/j.cellimm.2011.08.013. Epub 2011 Aug 30. Cell Immunol. 2011. PMID: 21939961
-
Cryptic epitope identified in rat and human cardiac myosin S2 region induces myocarditis in the Lewis rat.J Immunol. 2004 Mar 1;172(5):3225-34. doi: 10.4049/jimmunol.172.5.3225. J Immunol. 2004. PMID: 14978130
-
Cellular and molecular mechanisms of murine autoimmune myocarditis.APMIS. 1997 Jan;105(1):1-13. doi: 10.1111/j.1699-0463.1997.tb00532.x. APMIS. 1997. PMID: 9063494 Review.
-
Control of autoimmunity by "epitope theft".Trends Mol Med. 2005 Jan;11(1):1-4. doi: 10.1016/j.molmed.2004.11.002. Trends Mol Med. 2005. PMID: 15649815 Review.
Cited by
-
Circulating miR-185 might be a novel biomarker for clinical outcome in patients with dilated cardiomyopathy.Sci Rep. 2016 Sep 20;6:33580. doi: 10.1038/srep33580. Sci Rep. 2016. PMID: 27645404 Free PMC article.
-
Branched chain α-ketoacid dehydrogenase kinase 111-130, a T cell epitope that induces both autoimmune myocarditis and hepatitis in A/J mice.Immun Inflamm Dis. 2017 Dec;5(4):421-434. doi: 10.1002/iid3.177. Epub 2017 Jun 9. Immun Inflamm Dis. 2017. PMID: 28597552 Free PMC article.
-
Epitope Mapping of SERCA2a Identifies an Antigenic Determinant That Induces Mainly Atrial Myocarditis in A/J Mice.J Immunol. 2018 Jan 15;200(2):523-537. doi: 10.4049/jimmunol.1701090. Epub 2017 Dec 11. J Immunol. 2018. PMID: 29229678 Free PMC article.
-
β1-Adrenergic Receptor Contains Multiple IAk and IEk Binding Epitopes That Induce T Cell Responses with Varying Degrees of Autoimmune Myocarditis in A/J Mice.Front Immunol. 2017 Nov 20;8:1567. doi: 10.3389/fimmu.2017.01567. eCollection 2017. Front Immunol. 2017. PMID: 29209317 Free PMC article.
-
Viral myocarditis involves the generation of autoreactive T cells with multiple antigen specificities that localize in lymphoid and non-lymphoid organs in the mouse model of CVB3 infection.Mol Immunol. 2020 Aug;124:218-228. doi: 10.1016/j.molimm.2020.06.017. Epub 2020 Jun 29. Mol Immunol. 2020. PMID: 32615275 Free PMC article.
References
-
- Ken Murphy PT, Mark Walport. Immunobiology: The Immune System in Health and Disease. Taylor & Francis; New York: 2007.
-
- Guo HC, Jardetzky TS, Garrett TP, Lane WS, Strominger JL, Wiley DC. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature. 1992;360:364–6. - PubMed
-
- Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 6th Saunders Elsevier; Philadelphia: 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

