The role of Smad3 in the fibrotic phenotype in human vocal fold fibroblasts
- PMID: 26422444
- PMCID: PMC4814357
- DOI: 10.1002/lary.25673
The role of Smad3 in the fibrotic phenotype in human vocal fold fibroblasts
Abstract
Objectives/hypothesis: To investigate the role of Smad3 as a regulator of transforming growth factor (TGF)-β1-mediated cell activities associated with fibrosis in normal human vocal fold fibroblasts. We also sought to confirm the temporal stability of Smad3 knockdown via small inhibitor ribonucleic acid (siRNA). Vocal fold fibroblasts were employed to determine the effects of Smad3 knockdown on TGF-β1-mediated migration and contraction, as well as regulation of connective tissue growth factor (CTGF). We hypothesized that Smad3 is an ideal candidate for therapeutic manipulation in vivo based on its role in fibrosis.
Study design: In vitro.
Methods: Knockdown of Smad3 via siRNA was performed in our normal human vocal fold cell line. Three-dimensional collagen gel contraction and scratch assays were employed to determine the role of Smad3 on TGF-β1-mediated contraction and migration, respectively. The role Smad3 in the induction of CTGF was characterized via sodium dodecyl sulfate polyacrylamide gel electrophoresis. The effects of Smad3 signaling on Smad7 messenger (m)RNA and protein were also quantified.
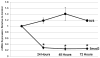
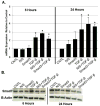
Results: Smad3 knockdown was temporally-stable up to 72 hours (P < 0.001), diminished TGF-β1-mediated collagen gel contraction and migration, and blunted induction of CTGF, but it had no effect on TGF-β1-mediated Smad7 mRNA or protein induction.
Conclusion: Transforming growth factor-β1 stimulated profibrotic cell activities in our cell line and these actions were largely reduced with Smad3 knockdown. These data provide continued support for therapeutic targeting of Smad3 for vocal fold fibrosis because it appears to regulate the fibrotic phenotype.
Level of evidence: N/A. Laryngoscope, 126:1151-1156, 2016.
Keywords: Smad3; TGF-β; Voice; fibrosis; scar; vocal fold.
© 2015 The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
No authors have any conflicts of interest or financial disclosures.
Figures
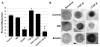
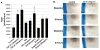
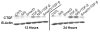
Similar articles
-
SMAD3 expression and regulation of fibroplasia in vocal fold injury.Laryngoscope. 2017 Sep;127(9):E308-E316. doi: 10.1002/lary.26648. Epub 2017 May 20. Laryngoscope. 2017. PMID: 28543554 Free PMC article.
-
Smad3: an emerging target for vocal fold fibrosis.Laryngoscope. 2014 Oct;124(10):2327-31. doi: 10.1002/lary.24723. Epub 2014 May 27. Laryngoscope. 2014. PMID: 24737245
-
Effects of transforming growth factor-beta1 on human vocal fold fibroblasts.Ann Otol Rhinol Laryngol. 2009 Mar;118(3):218-26. doi: 10.1177/000348940911800310. Ann Otol Rhinol Laryngol. 2009. PMID: 19374154
-
NR4A1 is an endogenous inhibitor of vocal fold fibrosis.Laryngoscope. 2017 Sep;127(9):E317-E323. doi: 10.1002/lary.26678. Epub 2017 Jun 5. Laryngoscope. 2017. PMID: 28581197 Free PMC article.
-
The Role of Cytokines in Modulating Vocal Fold Fibrosis: A Contemporary Review.Laryngoscope. 2021 Jan;131(1):139-145. doi: 10.1002/lary.28507. Epub 2020 Apr 15. Laryngoscope. 2021. PMID: 32293731 Review.
Cited by
-
Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability.Int J Mol Sci. 2019 May 24;20(10):2551. doi: 10.3390/ijms20102551. Int J Mol Sci. 2019. PMID: 31137626 Free PMC article. Review.
-
Antifibrotic effects of eupatilin on TGF-β1-treated human vocal fold fibroblasts.PLoS One. 2021 Mar 25;16(3):e0249041. doi: 10.1371/journal.pone.0249041. eCollection 2021. PLoS One. 2021. PMID: 33765087 Free PMC article.
-
Implementing Efficient Peptoid-Mediated Delivery of RNA-Based Therapeutics to the Vocal Folds.Laryngoscope Investig Otolaryngol. 2019 Oct 22;4(6):640-644. doi: 10.1002/lio2.310. eCollection 2019 Dec. Laryngoscope Investig Otolaryngol. 2019. PMID: 31890882 Free PMC article.
-
Stem Cell-Mediated Paracrine Signaling Alters Fibroplasia in Human Vocal Fold Fibroblasts in Vitro.Ann Otol Rhinol Laryngol. 2017 Aug;126(8):581-588. doi: 10.1177/0003489417716186. Epub 2017 Jun 21. Ann Otol Rhinol Laryngol. 2017. PMID: 28635301 Free PMC article.
-
Porcine Vocal Fold Lamina Propria-Derived Biomaterials Modulate TGF-β1-Mediated Fibroblast Activation in Vitro.ACS Biomater Sci Eng. 2020 Mar 9;6(3):1690-1703. doi: 10.1021/acsbiomaterials.9b01837. Epub 2020 Feb 11. ACS Biomater Sci Eng. 2020. PMID: 33455360 Free PMC article.
References
-
- Woodley DT, O’Keefe EJ, Prunieras M. Cutaneous wound healing: A model for cell-matrix interactions. J Am Acad Dermatol. 1985;12:420–433. - PubMed
-
- Varga J, Jimenez SA. Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Comm. 1986;138:974–980. - PubMed
-
- Ozbilgin MK, Inan S. The roles of transforming growth factor type beta (3) (TGF-beta(3)) and mast cells in the pathogenesis of scleroderma. Clin Rheumatol. 2003;22:189–195. - PubMed
-
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

