Surgical Intervention to Rescue Hirschsprung Disease in a Rat Model
- PMID: 26424040
- PMCID: PMC4622138
- DOI: 10.5056/jnm15079
Surgical Intervention to Rescue Hirschsprung Disease in a Rat Model
Abstract
Background/aims: Rats with a spontaneous null mutation in endothelin receptor type B or Ednrb (sl/sl; spotting lethal) lack enteric neurons in the distal bowel and usually die within the first week after birth. This early postnatal lethality limits their use for examining the potential of cell therapy to treat Hirschsprung disease, and for studies of the influence of EDNRB on the mature CNS and vascular systems.
Methods: We have developed a surgical intervention to prolong the life of the spotting lethal sl/sl rat, in which we perform a colostomy on postnatal (P) day 4-6 rats to avoid the fatal obstruction caused by the lack of colonic enteric neurons.
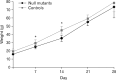
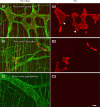
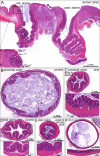
Results: The stomas remained patent and functional and the rats matured normally following surgery. Weight gains were comparable between control and Hirschsprung phenotype (sl/sl) rats, which were followed until 4 weeks after surgery (5 weeks old). We confirmed the absence of enteric neurons in the distal colon of rats whose lives were saved by the surgical intervention.
Conclusions: This study provides a novel approach for studying EDNRB signalling in multiple organ systems in mature rats, including an animal model to study the efficacy of cell therapy to treat Hirschsprung disease.
Keywords: Colostomy; Endothelin signalling; Enteric nervous system; Hirschsprung disease.
Figures

References
-
- Gershon MD. The Second Brain. New York: Harper Collins; 1998.
-
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

