An adverse event capture and management system for cancer studies
- PMID: 26424052
- PMCID: PMC4597098
- DOI: 10.1186/1471-2105-16-S13-S6
An adverse event capture and management system for cancer studies
Abstract
Background: Comprehensive capture of Adverse Events (AEs) is crucial for monitoring for side effects of a therapy while assessing efficacy. For cancer studies, the National Cancer Institute has developed the Common Terminology Criteria for Adverse Events (CTCAE) as a required standard for recording attributes and grading AEs. The AE assessments should be part of the Electronic Health Record (EHR) system; yet, due to patient-centric EHR design and implementation, many EHR's don't provide straightforward functions to assess ongoing AEs to indicate a resolution or a grade change for clinical trials.
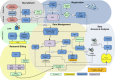
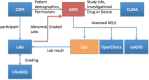
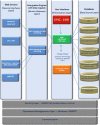
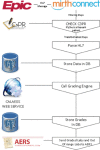
Methods: At UAMS, we have implemented a standards-based Adverse Event Reporting System (AERS) that is integrated with the Epic EHR and other research systems to track new and existing AEs, including automated lab result grading in a regulatory compliant manner. Within a patient's chart, providers can launch AERS, which opens the patient's ongoing AEs as default and allows providers to assess (resolution/ongoing) existing AEs. In another tab, it allows providers to create a new AE. Also, we have separated symptoms from diagnoses in the CTCAE to minimize inaccurate designation of the clinical observations. Upon completion of assessments, a physician would submit the AEs to the EHR via a Health Level 7 (HL7) message and then to other systems utilizing a Representational State Transfer Web Service.
Conclusions: AERS currently supports CTCAE version 3 and 4 with more than 65 cancer studies and 350 patients on those studies. This type of standard integrated into the EHR aids in research and data sharing in a compliant, efficient, and safe manner.
Figures



References
-
- CFR - Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr... http://www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr...
-
- Nass Sharyl J, Moses Harold L, Mendelsohn John. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: The National Academies Press; 2010. 3 Operations, Oversight, and Funding of Cancer Clinical Trials. - PubMed
-
- Rowin EJ, Lucier D, Pauker SG, Kumar S, Chen J, Salem DN. Does Error and Adverse Event Reporting by Physicians and Nurses Differ? Jt Comm J Qual Patient Saf. 2008;34:537–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

