Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): a multinational randomised trial
- PMID: 26424232
- PMCID: PMC4313760
- DOI: 10.1016/S2352-3018(14)00007-1
Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): a multinational randomised trial
Abstract
Background: Adherence is key to the success of antiretroviral therapy. Enhanced partner support might benefit patients with previous treatment failure. We aimed to assess whether an enhanced partner-based support intervention with modified directly observed therapy would improve outcomes with second-line therapy in HIV-infected patients for whom first-line therapy had failed.
Methods: We did a multicentre, international, randomised clinical trial at nine sites in Botswana, Brazil, Haiti, Peru, South Africa, Uganda, Zambia, and Zimbabwe. Participants aged 18 years or older for whom first-line therapy had failed, with HIV RNA concentrations greater than 1000 copies per mL and with a willing partner, were randomly assigned (1:1), via computer-generated randomisation, to receive partner-based modified directly observed therapy or standard of care. Randomisation was stratified by screening HIV RNA concentration (≤10 000 copies per mL vs >10 000 copies per mL). Participants and site investigators were not masked to group assignment. Primary outcome was confirmed virological failure (viral load >400 copies per mL) by week 48. Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00608569.
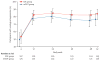
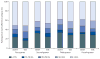
Findings: Between April 23, 2009, and Sept 29, 2011, we randomly assigned 259 participants to the modified directly observed therapy group (n=129) or the standard-of-care group (n=130). 34 (26%) participants in the modified directly observed therapy group achieved the primary endpoint of virological failure by week 48 compared with 23 (18%) participants in the standard-of-care group. The Kaplan-Meier estimated cumulative probability of virological failure by week 48 was 25·1% (95% CI 17·7-32·4) in the modified directly observed therapy group and 17·3% (10·8-23·7) in the standard-of-care group, for a weighted difference in standard of care versus modified directly observed therapy of -6·6% (95% CI -16·5% to 3·2%; p=0·19). 36 (14%) participants reported at least one grade 3 or higher adverse event or laboratory abnormality (n=21 in the modified directly observed therapy group and n=15 in the standard-of-care group).
Interpretation: Partner-based training with modified directly observed therapy had no effect on virological suppression. The intervention does not therefore seem to be a promising strategy to increase adherence. Intensive follow-up with clinic staff might be a viable approach in this setting.
Funding: AIDS Clinical Trials Group and the National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare that we have no conflicts of interest related to this work.
Comment in
-
Unlocking adherence: is gender the key?Lancet HIV. 2015 Jan;2(1):e2-3. doi: 10.1016/S2352-3018(14)00033-2. Epub 2014 Dec 11. Lancet HIV. 2015. PMID: 26424233 No abstract available.
References
-
- Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006 Aug 9;296(6):679–690. - PubMed
-
- Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Int Med. 2007 Apr 17;146(8):564–573. - PubMed
-
- Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002 Jul 13;360(9327):119–129. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 2UMIAI069456-08/PHS HHS/International
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1AI069434/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM 1AI069436/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- AI069438/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- UM1AI068636/AI/NIAID NIH HHS/United States
- U01 AI069438/AI/NIAID NIH HHS/United States
- U01-AI069467/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- UM1AI068634/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- AI069476/AI/NIAID NIH HHS/United States
- AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069438/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- 7UM1AI069455/AI/NIAID NIH HHS/United States
- AI 069421/AI/NIAID NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- U01-A1069501/PHS HHS/International
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UM1AI069481/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- 2UM1AI069438-08/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous