Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial
- PMID: 26424461
- PMCID: PMC4820828
- DOI: 10.1016/S2352-3018(14)00032-0
Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial
Abstract
Background: HIV-infected patients have a high risk of myocardial infarction. We aimed to assess the ability of statin treatment to reduce arterial inflammation and achieve regression of coronary atherosclerosis in this population.
Methods: In a randomised, double-blind, placebo-controlled trial, 40 HIV-infected participants with subclinical coronary atherosclerosis, evidence of arterial inflammation in the aorta by fluorodeoxyglucose (FDG)-PET, and LDL-cholesterol concentration of less than 3.37 mmol/L (130 mg/dL) were randomly assigned (1:1) to 1 year of treatment with atorvastatin or placebo. Randomisation was by the Massachusetts General Hospital (MGH) Clinical Research Pharmacy with a permuted-block algorithm, stratified by sex with a fixed block size of four. Study codes were available only to the MGH Research Pharmacy and not to study investigators or participants. The prespecified primary endpoint was arterial inflammation as assessed by FDG-PET of the aorta. Additional prespecified endpoints were non-calcified and calcified plaque measures and high risk plaque features assessed with coronary CT angiography and biochemical measures. Analysis was done by intention to treat with all available data and without imputation for missing data. The trial is registered with ClinicalTrials.gov, number NCT00965185.
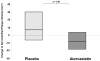
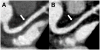
Findings: The study was done from Nov 13, 2009, to Jan 13, 2014. 19 patients were assigned to atorvastatin and 21 to placebo. 37 (93%) of 40 participants completed the study, with equivalent discontinuation rates in both groups. Baseline characteristics were similar between groups. After 12 months, change in FDG-PET uptake of the most diseased segment of the aorta was not different between atorvastatin and placebo, but technically adequate results comparing longitudinal changes in identical regions could be assessed in only 21 patients (atorvastatin Δ -0.03, 95% CI -0.17 to 0.12, vs placebo Δ -0.06, -0.25 to 0.13; p=0.77). Change in plaque could be assessed in all 37 people completing the study. Atorvastatin reduced non-calcified coronary plaque volume relative to placebo: median change -19.4% (IQR -39.2 to 9.3) versus 20.4% (-7.1 to 94.4; p=0.009, n=37). The number of high-risk plaques was significantly reduced in the atorvastatin group compared with the placebo group: change in number of low attenuation plaques -0.2 (95% CI -0.6 to 0.2) versus 0.4 (0.0, 0.7; p=0.03; n=37); and change in number of positively remodelled plaques -0.2 (-0.4 to 0.1) versus 0.4 (-0.1 to 0.8; p=0.04; n=37). Direct LDL-cholesterol (-1.00 mmol/L, 95% CI -1.38 to 0.61 vs 0.30 mmol/L, 0.04 to 0.55, p<0.0001) and lipoprotein-associated phospholipase A2 (-52.2 ng/mL, 95% CI -70.4 to -34.0, vs -13.3 ng/mL, -32.8 to 6.2; p=0.005; n=37) decreased significantly with atorvastatin relative to placebo. Statin therapy was well tolerated, with a low incidence of clinical adverse events.
Interpretation: No significant effects of statin therapy on arterial inflammation of the aorta were seen as measured by FDG-PET. However, statin therapy reduced non-calcified plaque volume and high-risk coronary plaque features in HIV-infected patients. Further studies should assess whether reduction in high-risk coronary artery disease translates into effective prevention of cardiovascular events in this at-risk population.
Funding: National Institutes of Health, Harvard Clinical and Translational Science Center, National Center for Research Resources.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr. Grinspoon has consulted with Navidea, AstraZeneca, NovoNordisk, and Theratechnologies, and received grant support from Gilead, Amgen, KOWA Pharmaceuticals, and Theratechnologies, unrelated to this manuscript. Dr. Robbins has received grant support from Gilead Sciences unrelated to this manuscript. Dr. Hoffmann received grant support from Siemens Healthcare, American College of Radiology Imaging Network, and HeartFlow Inc., unrelated to this manuscript. Dr. Tawakol has received grant support from Genentech/Roche, BMS, Takeda, GSK, and VBL and personal fees from Novartis, Genentech/Roche, BMS, Cerenis, Takeda, Actelion, GSK, and Amgen unrelated to this manuscript. All authors report no conflicts of interest.
Figures

Comment in
-
Should everyone ageing with HIV take a statin?Lancet HIV. 2015 Feb;2(2):e36-7. doi: 10.1016/S2352-3018(14)00062-9. Epub 2015 Jan 9. Lancet HIV. 2015. PMID: 26424457 No abstract available.
References
-
- Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44(12):1625–31. - PubMed
-
- Lohse N, Hansen A-BE, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of Persons with and without HIV Infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. - PubMed
-
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

