Effect of therapeutic intensification followed by HIV DNA prime and rAd5 boost vaccination on HIV-specific immunity and HIV reservoir (EraMune 02): a multicentre randomised clinical trial
- PMID: 26424549
- PMCID: PMC6322849
- DOI: 10.1016/S2352-3018(15)00026-0
Effect of therapeutic intensification followed by HIV DNA prime and rAd5 boost vaccination on HIV-specific immunity and HIV reservoir (EraMune 02): a multicentre randomised clinical trial
Abstract
Background: Achievement of a cure for HIV infection might need reactivation of latent virus and improvement of HIV-specific immunity. As an initial step, in this trial we assessed the effect of antiretroviral therapy intensification and immune modulation with a DNA prime and recombinant adenovirus 5 (rAd5) boost vaccine.
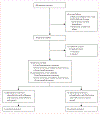
Methods: In this multicentre, randomised, open-label, non-comparative, phase 2 clinical trial, we enrolled eligible adults 18-70 years of age with chronic HIV-1 infection on suppressive antiretroviral therapy with current CD4 count of at least 350 cells per μL and HIV DNA between 10 and 1000 copies per 10(6) peripheral blood mononuclear cells. After an 8 week lead-in of antiretroviral intensification therapy (standard dose raltegravir and dose-adjusted maraviroc based on baseline antiretroviral therapy), patients were randomly assigned (1:1) to receive antiretroviral therapy intensification alone or intensification plus injections of HIV DNA prime vaccine (4 mg VRC-HIVDNA016-00-VP) at weeks 8, 12, and 16, followed by HIV rAd5 boost vaccine (10(10) particle units of VRC-HIVADV014-00-VP) at week 32. Randomisation was computer generated in permuted blocks of six and was stratified by study site. The primary endpoint was a 0·5 log10 or greater decrease in HIV DNA in peripheral blood mononuclear cells at week 56. This study is registered with ClinicalTrials.gov, number NCT00976404.
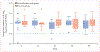
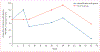
Findings: Between Nov 29, 2010, and Oct 28, 2011, we enrolled 28 eligible patients from three academic HIV clinics in the USA. After the 8 week lead-in of antiretroviral intensification therapy, 14 patients were randomly assigned to continue antiretroviral therapy intensification alone and 14 to intensification plus vaccine. Enrolled participants had median CD4 count of 636 cells per μL, median HIV DNA 170 copies per 10(6) peripheral blood mononuclear cells, and duration of antiretroviral therapy of 13 years. The median amount of HIV DNA did not change significantly between baseline and week 56 in the antiretroviral therapy intensification plus vaccine group. One participant in the antiretroviral therapy intensification alone group reached the primary endpoint, with 0·55 log10 decrease in HIV DNA in peripheral blood mononuclear cells. Both treatments were well tolerated. No severe or systemic reactions to vaccination occurred, and five serious adverse events were recorded during the study, most of which resolved spontaneously or were judged unrelated to study treatments.
Interpretation: Antiretroviral therapy intensification followed by DNA prime and rAd5 boost vaccine did not significantly increase HIV expression or reduce the latent HIV reservoir. A multifaceted approach that includes stronger activators of HIV expression and novel immune modulators will probably be needed to reduce the latent HIV reservoir and allow for long-term control in patients off antiretroviral therapy.
Funding: Objectif Recherche Vaccin SIDA (ORVACS).
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Trying to cure HIV with immunotherapy: not so simple.Lancet HIV. 2015 Mar;2(3):e72-3. doi: 10.1016/S2352-3018(15)00022-3. Epub 2015 Feb 17. Lancet HIV. 2015. PMID: 26424544 No abstract available.
References
-
- Buzón MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 2010; 16: 460–65. - PubMed
-
- Shete A, Thakar M, Singh DP, et al. Short communication: HIV antigen-specific reactivation of HIV infection from cellular reservoirs: implications in the settings of therapeutic vaccinations. AIDS Res Hum Retroviruses 2012; 28: 835–43. - PubMed
-
- Patterson LJ, Kuate S, Daltabuit-Test M, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) vectors efciently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin Vaccine Immunol 2012; 19: 629–37. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

