The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry
- PMID: 26425178
- PMCID: PMC4578405
- DOI: 10.3762/bjoc.11.134
The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry
Abstract
The implementation of continuous flow processing as a key enabling technology has transformed the way we conduct chemistry and has expanded our synthetic capabilities. As a result many new preparative routes have been designed towards commercially relevant drug compounds achieving more efficient and reproducible manufacture. This review article aims to illustrate the holistic systems approach and diverse applications of flow chemistry to the preparation of pharmaceutically active molecules, demonstrating the value of this strategy towards every aspect ranging from synthesis, in-line analysis and purification to final formulation and tableting. Although this review will primarily concentrate on large scale continuous processing, additional selected syntheses using micro or meso-scaled flow reactors will be exemplified for key transformations and process control. It is hoped that the reader will gain an appreciation of the innovative technology and transformational nature that flow chemistry can leverage to an overall process.
Keywords: continuous processing; flow synthesis; in-line analysis; manufacture; pharmaceuticals; scalability.
Figures
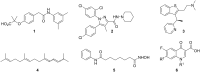
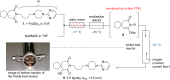
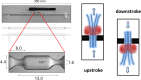

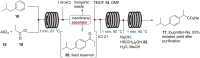
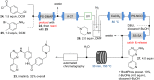
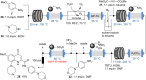
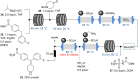
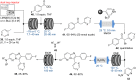
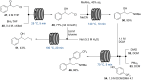
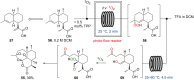
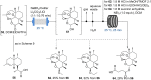

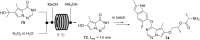


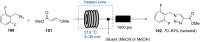

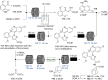
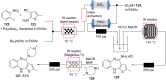
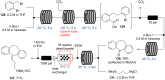
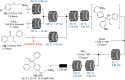

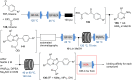

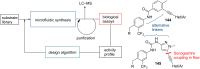
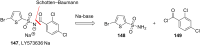
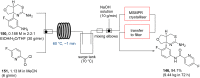
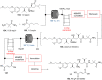
References
-
- Wegner J, Ceylan S, Kirschning A. Adv Synth Catal. 2012;354:17–57. doi: 10.1002/adsc.201100584. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
