Over-expression of XIST, the Master Gene for X Chromosome Inactivation, in Females With Major Affective Disorders
- PMID: 26425698
- PMCID: PMC4563114
- DOI: 10.1016/j.ebiom.2015.06.012
Over-expression of XIST, the Master Gene for X Chromosome Inactivation, in Females With Major Affective Disorders
Abstract
Background: Psychiatric disorders are common mental disorders without a pathological biomarker. Classic genetic studies found that an extra X chromosome frequently causes psychiatric symptoms in patients with either Klinefelter syndrome (XXY) or Triple X syndrome (XXX). Over-dosage of some X-linked escapee genes was suggested to cause psychiatric disorders. However, relevance of these rare genetic diseases to the pathogenesis of psychiatric disorders in the general population of psychiatric patients is unknown.
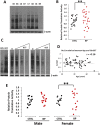
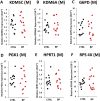
Methods: XIST and several X-linked genes were studied in 36 lymphoblastoid cell lines from healthy females and 60 lymphoblastoid cell lines from female patients with either bipolar disorder or recurrent major depression. XIST and KDM5C expression was also quantified in 48 RNA samples from postmortem human brains of healthy female controls and female psychiatric patients.
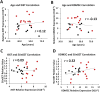
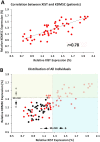
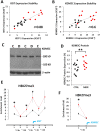
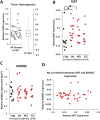
Findings: We found that the XIST gene, a master in control of X chromosome inactivation (XCI), is significantly over-expressed (p = 1 × 10(- 7), corrected after multiple comparisons) in the lymphoblastoid cells of female patients with either bipolar disorder or major depression. The X-linked escapee gene KDM5C also displays significant up-regulation (p = 5.3 × 10(- 7), corrected after multiple comparisons) in the patients' cells. Expression of XIST and KDM5C is highly correlated (Pearson's coefficient, r = 0.78, p = 1.3 × 10(- 13)). Studies on human postmortem brains supported over-expression of the XIST gene in female psychiatric patients.
Interpretations: We propose that over-expression of XIST may cause or result from subtle alteration of XCI, which up-regulates the expression of some X-linked escapee genes including KDM5C. Over-expression of X-linked genes could be a common mechanism for the development of psychiatric disorders between patients with those rare genetic diseases and the general population of female psychiatric patients with XIST over-expression. Our studies suggest that XIST and KDM5C expression could be used as a biological marker for diagnosis of psychiatric disorders in a significantly large subset of female patients.
Research in context: Due to lack of biological markers, diagnosis and treatment of psychiatric disorders are subjective. There is utmost urgency to identify biomarkers for clinics, research, and drug development. We found that XIST and KDM5C gene expression may be used as a biological marker for diagnosis of major affective disorders in a significantly large subset of female patients from the general population. Our studies show that over-expression of XIST and some X-linked escapee genes may be a common mechanism for development of psychiatric disorders between the patients with rare genetic diseases (XXY or XXX) and the general population of female psychiatric patients.
Keywords: KDM5C; Major affective disorders; X chromosome inactivation; X-linked escapee genes; XIST.
Figures






References
-
- Amir R.E., Van den Veyver I.B., Schultz R. Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann. Neurol. 2000;47(5):670–679. - PubMed
-
- Bender B.G., Linden M.G., Harmon R.J. Neuropsychological and functional cognitive skills of 35 unselected adults with sex chromosome abnormalities. Am. J. Med. Genet. 2001;102(4):309–313. - PubMed
-
- Brown C.J., Hendrich B.D., Rupert J.L. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71(3):527–542. - PubMed
-
- Buzin C.H., Mann J.R., Singer-Sam J. Quantitative RT-PCR assays show Xist RNA levels are low in mouse female adult tissue, embryos and embryoid bodies. Development. 1994;120(12):3529–3536. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

