The Effect of Radiation Dose and Variation in Neupogen® Initiation Schedule on the Mitigation of Myelosuppression during the Concomitant GI-ARS and H-ARS in a Nonhuman Primate Model of High-dose Exposure with Marrow Sparing
- PMID: 26425903
- PMCID: PMC9442798
- DOI: 10.1097/HP.0000000000000350
The Effect of Radiation Dose and Variation in Neupogen® Initiation Schedule on the Mitigation of Myelosuppression during the Concomitant GI-ARS and H-ARS in a Nonhuman Primate Model of High-dose Exposure with Marrow Sparing
Abstract
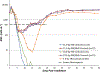
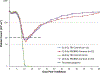
A nonhuman primate (NHP) model of acute high-dose, partial-body irradiation with 5% bone marrow (PBI/BM5) sparing was used to assess the effect of Neupogen® [granulocyte colony stimulating factor (G-CSF)] to mitigate the associated myelosuppression when administered at an increasing interval between exposure and initiation of treatment. A secondary objective was to assess the effect of Neupogen® on the mortality or morbidity of the hematopoietic (H)- acute radiation syndrome (ARS) and concurrent acute gastrointestinal radiation syndrome (GI-ARS). NHP were exposed to 10.0 or 11.0 Gy with 6 MV LINAC-derived photons at approximately 0.80 Gy min. All NHP received medical management. NHP were dosed daily with control article (5% dextrose in water) initiated on day 1 post-exposure or Neupogen® (10 μg kg) initiated on day 1, day 3, or day 5 until recovery [absolute neutrophil count (ANC) ≥ 1,000 cells μL for three consecutive days]. Mortality in both the 10.0 Gy and 11.0 Gy cohorts suggested that early administration of Neupogen® at day 1 post exposure may affect acute GI-ARS mortality, while Neupogen® appeared to mitigate mortality due to the H-ARS. However, the study was not powered to detect statistically significant differences in survival. The ability of Neupogen® to stimulate granulopoiesis was assessed by evaluating key parameters for ANC recovery: the depth of nadir, duration of neutropenia (ANC < 500 cells μL) and recovery time to ANC ≥ 1,000 cells μL. Following 10.0 Gy PBI/BM5, the mean duration of neutropenia was 11.6 d in the control cohort vs. 3.5 d and 4.6 d in the day 1 and day 3 Neupogen® cohorts, respectively. The respective ANC nadirs were 94 cells μL, 220 cells μL, and 243 cells μL for the control and day 1 and day 3 Neupogen® cohorts. Following 11.0 Gy PBI/BM5, the duration of neutropenia was 10.9 d in the control cohort vs. 2.8 d, 3.8 d, and 4.5 d in the day 1, day 3, and day 5 Neupogen® cohorts, respectively. The respective ANC nadirs for the control and day 1, day 3, and day 5 Neupogen® cohorts were 131 cells μL, 292 cells μL, 236 cells μL, and 217 cells μL, respectively. Therefore, the acceleration of granulopoiesis by Neupogen® in this model is independent of the time interval between radiation exposure and treatment initiation up to 5 d post-exposure. The PBI/BM5 model can be used to assess medical countermeasure efficacy in the context of the concurrent GI- and H-ARS.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflicts of interest.
Figures





References
-
- Baranov AE. Allogenic Bone Marrow Transplantation After Severe, Uniform Total-Body Irradiation: Experience From Recent (Nyasvizh, Belarus) and Previous Radiation Accidents. In: MacVittie TJ, Weiss JF, Browne D, eds. Advances in the Treatment of Radiation Injuries: Advances in the Biosciences, Vol. 94. Tarrytown, NY: Pergamon; 1996: 281–293.
-
- Baranov AE and Guskova AK. Acute radiation disease in Chernobyl accident victims. In: Ricks RC, Fry SA, eds. The medical basis for radiation accident preparedness II; Clinical Experience and follow-up since 1979. New York: Elsevier; 1990: 79–87.
-
- Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, Gorin C, Thierry D, Gourmelon P. Comparison of Autologous Cell Therapy and Granulocyte colony-stimulating Factor (G-CSF) Injection vs G-CSF Alone for the Treatment of Acute Radiation Syndrome in a Nonhuman Primate Model. Int J Radiat Oncol Biol Phys 63:911–920; 2005a. - PubMed
-
- Bertho JM, Prat M, Frick J, Demarquay C, Gaugler MH, Dudoignon N, Clairand I, Chapel A, Gorin NC, Thierry D. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res 163:557–570; 2005b. - PubMed
-
- Bond VP, Carsten AL, Bullis J, Roth SP. Severity of Organ Injury as a Predictor of Acute Mortality for Disparate Patterns of Absorbed Dose Distribution. Radiat Res 128:S9–S11; 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

