Biomechanics of Cardiac Function
- PMID: 26426462
- PMCID: PMC4668273
- DOI: 10.1002/cphy.c140070
Biomechanics of Cardiac Function
Abstract
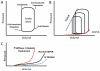
The heart pumps blood to maintain circulation and ensure the delivery of oxygenated blood to all the organs of the body. Mechanics play a critical role in governing and regulating heart function under both normal and pathological conditions. Biological processes and mechanical stress are coupled together in regulating myocyte function and extracellular matrix structure thus controlling heart function. Here, we offer a brief introduction to the biomechanics of left ventricular function and then summarize recent progress in the study of the effects of mechanical stress on ventricular wall remodeling and cardiac function as well as the effects of wall mechanical properties on cardiac function in normal and dysfunctional hearts. Various mechanical models to determine wall stress and cardiac function in normal and diseased hearts with both systolic and diastolic dysfunction are discussed. The results of these studies have enhanced our understanding of the biomechanical mechanism in the development and remodeling of normal and dysfunctional hearts. Biomechanics provide a tool to understand the mechanism of left ventricular remodeling in diastolic and systolic dysfunction and guidance in designing and developing new treatments.
Copyright © 2015 John Wiley & Sons, Inc.
Figures

References
-
- Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JAC, Smiseth OA, Slørdahl SA. Noninvasive Myocardial Strain Measurement by Speckle Tracking Echocardiography: Validation Against Sonomicrometry and Tagged Magnetic Resonance Imaging. Journal of the American College of Cardiology. 2006;47:789–793. - PubMed
-
- Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J. 2013;77:580–587. - PubMed
-
- Aronson D, Musallam A, Lessick J, Dabbah S, Carasso S, Hammerman H, Reisner S, Agmon Y, Mutlak D. Impact of diastolic dysfunction on the development of heart failure in diabetic patients after acute myocardial infarction. Circ Heart Fail. 2010;3:125–131. - PubMed
-
- Ashraf M, Myronenko A, Nguyen T, Inage A, Smith W, Lowe RI, Thiele K, Gibbons Kroeker CA, Tyberg JV, Smallhorn JF, Sahn DJ, Song X. Defining left ventricular apex-to-base twist mechanics computed from high-resolution 3D echocardiography: validation against sonomicrometry. JACC Cardiovasc Imaging. 2010;3:227–234. - PubMed
Further Reading
-
- Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer; New York: 2002.
-
- Fung YC. Biomechanics: Circulation. 2nd Ed. Springer; New York: 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

