Physiological Implications of Myocardial Scar Structure
- PMID: 26426470
- PMCID: PMC4727398
- DOI: 10.1002/cphy.c140067
Physiological Implications of Myocardial Scar Structure
Abstract
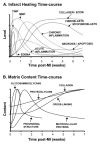
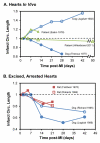
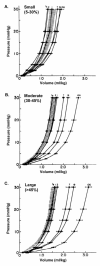
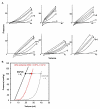
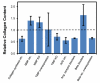
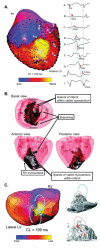
Once myocardium dies during a heart attack, it is replaced by scar tissue over the course of several weeks. The size, location, composition, structure, and mechanical properties of the healing scar are all critical determinants of the fate of patients who survive the initial infarction. While the central importance of scar structure in determining pump function and remodeling has long been recognized, it has proven remarkably difficult to design therapies that improve heart function or limit remodeling by modifying scar structure. Many exciting new therapies are under development, but predicting their long-term effects requires a detailed understanding of how infarct scar forms, how its properties impact left ventricular function and remodeling, and how changes in scar structure and properties feed back to affect not only heart mechanics but also electrical conduction, reflex hemodynamic compensations, and the ongoing process of scar formation itself. In this article, we outline the scar formation process following a myocardial infarction, discuss interpretation of standard measures of heart function in the setting of a healing infarct, then present implications of infarct scar geometry and structure for both mechanical and electrical function of the heart and summarize experiences to date with therapeutic interventions that aim to modify scar geometry and structure. One important conclusion that emerges from the studies reviewed here is that computational modeling is an essential tool for integrating the wealth of information required to understand this complex system and predict the impact of novel therapies on scar healing, heart function, and remodeling following myocardial infarction.
Copyright © 2015 John Wiley & Sons, Inc.
Figures



References
-
- Amigoni M, Meris A, Thune JJ, Mangalat D, Skali H, Bourgoun M, Warnica JW, Barvik S, Arnold JMO, Velazquez EJ, Van de Werf F, Ghali J, McMurray JJV, Køber L, Pfeffer M, Solomon SD. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J. 2007;28:326–33. - PubMed
-
- Antoni ML, Boden H, Hoogslag GE, Ewe SH, Auger D, Holman ER, van der Wall EE, Schalij MJ, Bax JJ, Delgado V. Prevalence of dyssynchrony and relation with long-term outcome in patients after acute myocardial infarction. Am J Cardiol. 2011;108:1689–96. - PubMed
-
- Aronson D, Goldsher N, Zukermann R, Kapeliovich M, Lessick J, Mutlak D, Dabbah S, Markiewicz W, Beyar R, Hammerman H, Reisner S, Agmon Y. Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med. 2006;166:2362–8. - PubMed
-
- Arruda M, Fahmy T, Armaganijan L, Di Biase L, Patel D, Natale A. Endocardial and epicardial mapping and catheter ablation of post myocardial infarction ventricular tachycardia: A substrate modification approach. J Interv Card Electrophysiol. 2010;28:137–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

