Protection from Radiation-Induced Pulmonary Fibrosis by Peripheral Targeting of Cannabinoid Receptor-1
- PMID: 26426981
- PMCID: PMC4742897
- DOI: 10.1165/rcmb.2014-0331OC
Protection from Radiation-Induced Pulmonary Fibrosis by Peripheral Targeting of Cannabinoid Receptor-1
Abstract
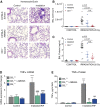
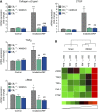
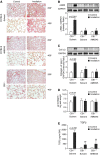
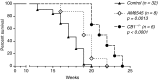
Radiation-induced pulmonary fibrosis (RIF) is a severe complication of thoracic radiotherapy that limits its dose, intensity, and duration. The contribution of the endocannabinoid signaling system in pulmonary fibrogenesis is not known. Using a well-established mouse model of RIF, we assessed the involvement of cannabinoid receptor-1 (CB1) in the onset and progression of pulmonary fibrosis. Female C57BL/6 mice and CB1 knockout mice generated on C57BL/6 background received 20 Gy (2 Gy/min) single-dose thoracic irradiation that resulted in pulmonary fibrosis and animal death within 15 to 18 weeks. Some C57BL/6 animals received the CB1 peripherally restricted antagonist AM6545 at 1 mg/kg intraperitoneally three times per week. Animal survival and parameters of pulmonary inflammation and fibrosis were evaluated. Thoracic irradiation (20 Gy) was associated with marked pulmonary inflammation and fibrosis in mice and high mortality within 15 to 18 weeks after exposure. Genetic deletion or pharmacological inhibition of CB1 receptors with a peripheral CB1 antagonist AM6545 markedly attenuated or delayed the lung inflammation and fibrosis and increased animal survival. Our results show that CB1 signaling plays a key pathological role in the development of radiation-induced pulmonary inflammation and fibrosis, and peripherally restricted CB1 antagonists may represent a novel therapeutic approach against this devastating complication of radiotherapy/irradiation.
Keywords: AM6545; cannabinoid receptor 1; peripherally restricted CB1 antagonist; radiation-induced pulmonary fibrosis; thoracic irradiation.
Figures

References
-
- Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13:242–248. - PubMed
-
- Yu TK, Whitman GJ, Thames HD, Buzdar AU, Strom EA, Perkins GH, Schechter NR, McNeese MD, Kau SW, Thomas ES, et al. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst. 2004;96:1676–1681. - PubMed
-
- Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. - PubMed
-
- Marnitz S, Garcia-Gonzalez E, Selvi E, Akhmetshina A, Palumbo K, Lorenzini S, Maggio R, Lucattelli M, Galeazzi M, Distler JW. Long-term results of total body irradiation in adults with acute lymphoblastic leukemia. Strahlenther Onkol. 2014;190:453–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

