Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance
- PMID: 26428501
- PMCID: PMC4774647
- DOI: 10.1016/j.ajog.2015.08.040
Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance
Abstract
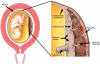
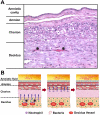
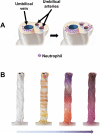
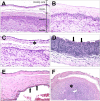
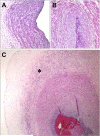
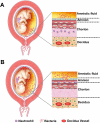
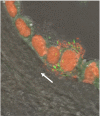
Acute inflammatory lesions of the placenta consist of diffuse infiltration of neutrophils at different sites in the organ. These lesions include acute chorioamnionitis, funisitis, and chorionic vasculitis and represent a host response (maternal or fetal) to a chemotactic gradient in the amniotic cavity. While acute chorioamnionitis is evidence of a maternal host response, funisitis and chorionic vasculitis represent fetal inflammatory responses. Intraamniotic infection generally has been considered to be the cause of acute chorioamnionitis and funisitis; however, recent evidence indicates that "sterile" intraamniotic inflammation, which occurs in the absence of demonstrable microorganisms induced by "danger signals," is frequently associated with these lesions. In the context of intraamniotic infection, chemokines (such as interleukin-8 and granulocyte chemotactic protein) establish a gradient that favors the migration of neutrophils from the maternal or fetal circulation into the chorioamniotic membranes or umbilical cord, respectively. Danger signals that are released during the course of cellular stress or cell death can also induce the release of neutrophil chemokines. The prevalence of chorioamnionitis is a function of gestational age at birth, and present in 3-5% of term placentas and in 94% of placentas delivered at 21-24 weeks of gestation. The frequency is higher in patients with spontaneous labor, preterm labor, clinical chorioamnionitis (preterm or term), or ruptured membranes. Funisitis and chorionic vasculitis are the hallmarks of the fetal inflammatory response syndrome, a condition characterized by an elevation in the fetal plasma concentration of interleukin-6, and associated with the impending onset of preterm labor, a higher rate of neonatal morbidity (after adjustment for gestational age), and multiorgan fetal involvement. This syndrome is the counterpart of the systemic inflammatory response syndrome in adults: a risk factor for short- and long-term complications (ie, sterile inflammation in fetuses, neonatal sepsis, bronchopulmonary dysplasia, periventricular leukomalacia, and cerebral palsy). This article reviews the definition, pathogenesis, grading and staging, and clinical significance of the most common lesions in placental disease. Illustrations of the lesions and diagrams of the mechanisms of disease are provided.
Keywords: CXCL6; chorionic vasculitis; fetal inflammatory response syndrome; granulocyte chemotactic protein; interleukin (IL)-8; microbial invasion of the amniotic cavity; placental pathology; pregnancy; prematurity; preterm; staging; sterile inflammation.
Published by Elsevier Inc.
Figures



Comment in
-
Placental inflammation, neonatal death and cerebral palsy in preterm infants: is there a relationship?BJOG. 2016 Nov;123(12):1964. doi: 10.1111/1471-0528.14210. Epub 2016 Jul 18. BJOG. 2016. PMID: 27428550 No abstract available.
References
-
- Blanc WA. Amniotic infection syndrome; pathogenesis, morphology, and significance in circumnatal mortality. Clin Obstet Gynecol. 1959;2:705–34. - PubMed
-
- Russell P. Inflammatory lesions of the human placenta: Clinical significance of acute chorioamnionitis. Am J Diagn Gynecol Obstet. 1979;2:127–37.
-
- Blanc WA. Pathology of the placenta and cord in ascending and in haematogenous infection. Ciba Found Symp. 1979;(77):17–38. - PubMed
-
- Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319(15):972–8. - PubMed
-
- Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. Sixth ed. Springer; Berlin Heidelberg: 2012. Infectious Diseases. pp. 557–656.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

