On scattered waves and lipid domains: detecting membrane rafts with X-rays and neutrons
- PMID: 26428538
- PMCID: PMC4719199
- DOI: 10.1039/c5sm01807b
On scattered waves and lipid domains: detecting membrane rafts with X-rays and neutrons
Abstract
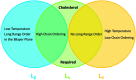
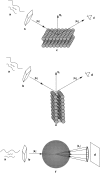
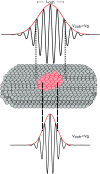
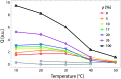
In order to understand the biological role of lipids in cell membranes, it is necessary to determine the mesoscopic structure of well-defined model membrane systems. Neutron and X-ray scattering are non-invasive, probe-free techniques that have been used extensively in such systems to probe length scales ranging from angstroms to microns, and dynamics occurring over picosecond to millisecond time scales. Recent developments in the area of phase separated lipid systems mimicking membrane rafts will be presented, and the underlying concepts of the different scattering techniques used to study them will be discussed in detail.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

