IMAGE ARTIFACTS IN OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY
- PMID: 26428607
- PMCID: PMC4712934
- DOI: 10.1097/IAE.0000000000000765
IMAGE ARTIFACTS IN OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY
Abstract
Purpose: To describe image artifacts of optical coherence tomography (OCT) angiography and their underlying causative mechanisms. To establish a common vocabulary for the artifacts observed.
Methods: The methods by which OCT angiography images are acquired, generated, and displayed are reviewed as are the mechanisms by which each or all of these methods can produce extraneous image information. A common set of terminology is proposed and used.
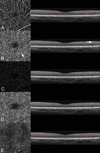
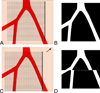
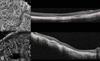
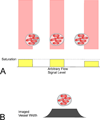
Results: Optical coherence tomography angiography uses motion contrast to image blood flow and thereby images the vasculature without the need for a contrast agent. Artifacts are very common and can arise from the OCT image acquisition, intrinsic characteristics of the eye, eye motion, image processing, and display strategies. Optical coherence tomography image acquisition for angiography takes more time than simple structural scans and necessitates trade-offs in flow resolution, scan quality, and speed. An important set of artifacts are projection artifacts in which images of blood vessels seem at erroneous locations. Image processing used for OCT angiography can alter vascular appearance through segmentation defects, and because of image display strategies can give false impressions of the density and location of vessels. Eye motion leads to discontinuities in displayed data. Optical coherence tomography angiography artifacts can be detected by interactive evaluation of the images.
Conclusion: Image artifacts are common and can lead to incorrect interpretations of OCT angiography images. Because of the quantity of data available and the potential for artifacts, physician interaction in viewing the image data will be required, much like what happens in modern radiology practice.
Figures


















References
-
- Fingler J, Schwartz D, Yang C, Fraser SE. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt Express. 2007;15(20):12636–12653. - PubMed
-
- Fingler J, Readhead C, Schwartz DM, Fraser SE. Phase-contrast OCT imaging of transverse flows in the mouse retina and choroid. Invest Ophthalmol Vis Sci. 2008;49(11):5055–5059. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

