Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies
- PMID: 26428619
- PMCID: PMC4698222
- DOI: 10.1016/j.addr.2015.09.010
Engineered heart tissues and induced pluripotent stem cells: Macro- and microstructures for disease modeling, drug screening, and translational studies
Abstract
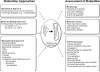
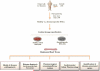
Engineered heart tissue has emerged as a personalized platform for drug screening. With the advent of induced pluripotent stem cell (iPSC) technology, patient-specific stem cells can be developed and expanded into an indefinite source of cells. Subsequent developments in cardiovascular biology have led to efficient differentiation of cardiomyocytes, the force-producing cells of the heart. iPSC-derived cardiomyocytes (iPSC-CMs) have provided potentially limitless quantities of well-characterized, healthy, and disease-specific CMs, which in turn has enabled and driven the generation and scale-up of human physiological and disease-relevant engineered heart tissues. The combined technologies of engineered heart tissue and iPSC-CMs are being used to study diseases and to test drugs, and in the process, have advanced the field of cardiovascular tissue engineering into the field of precision medicine. In this review, we will discuss current developments in engineered heart tissue, including iPSC-CMs as a novel cell source. We examine new research directions that have improved the function of engineered heart tissue by using mechanical or electrical conditioning or the incorporation of non-cardiomyocyte stromal cells. Finally, we discuss how engineered heart tissue can evolve into a powerful tool for therapeutic drug testing.
Keywords: Cardiovascular disease; Disease modeling; Drug screening; Induced pluripotent stem cells; Tissue engineering.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

References
-
- Laslett LJ, Alagona P, Jr., Clark BA, 3rd, Drozda JP, Jr., Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–49. - PubMed
-
- Herper M. The cost of creating a new drug now $5 billion, pushing big pharma to change. Forbes.com. 2013
-
- Desmond-Hellmann S. The Cost of Creating A New Drug Now $5 Billiion. Pushing Big Pharma To Change, Forbes. 2013
-
- Engle SJ, Puppala D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell. 2013;12:669–677. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

