Molecular Mechanisms and Kinetic Effects of FXYD1 and Phosphomimetic Mutants on Purified Human Na,K-ATPase
- PMID: 26429909
- PMCID: PMC4661392
- DOI: 10.1074/jbc.M115.687913
Molecular Mechanisms and Kinetic Effects of FXYD1 and Phosphomimetic Mutants on Purified Human Na,K-ATPase
Abstract
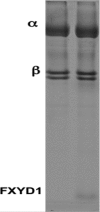
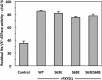
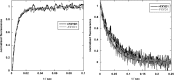
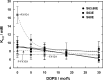
Phospholemman (FXYD1) is a single-transmembrane protein regulator of Na,K-ATPase, expressed strongly in heart, skeletal muscle, and brain and phosphorylated by protein kinases A and C at Ser-68 and Ser-63, respectively. Binding of FXYD1 reduces Na,K-ATPase activity, and phosphorylation at Ser-68 or Ser-63 relieves the inhibition. Despite the accumulated information on physiological effects, whole cell studies provide only limited information on molecular mechanisms. As a complementary approach, we utilized purified human Na,K-ATPase (α1β1 and α2β1) reconstituted with FXYD1 or mutants S63E, S68E, and S63E,S68E that mimic phosphorylation at Ser-63 and Ser-68. Compared with control α1β1, FXYD1 reduces Vmax and turnover rate and raises K0.5Na. The phosphomimetic mutants reverse these effects and reduce K0.5Na below control K0.5Na. Effects on α2β1 are similar but smaller. Experiments in proteoliposomes reconstituted with α1β1 show analogous effects of FXYD1 on K0.5Na, which are abolished by phosphomimetic mutants and also by increasing mole fractions of DOPS in the proteoliposomes. Stopped-flow experiments using the dye RH421 show that FXYD1 slows the conformational transition E2(2K)ATP → E1(3Na)ATP but does not affect 3NaE1P → E2P3Na. This regulatory effect is explained simply by molecular modeling, which indicates that a cytoplasmic helix (residues 60-70) docks between the αN and αP domains in the E2 conformation, but docking is weaker in E1 (also for phosphomimetic mutants). Taken together with previous work showing that FXYD1 also raises binding affinity for the Na(+)-selective site III, these results provide a rather comprehensive picture of the regulatory mechanism of FXYD1 that complements the physiological studies.
Keywords: FXYD protein; Na+/K+-ATPase; docking; fluorescence; kinetics; membrane protein; phosphomimetic mutant; regulatory mechanism.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Blanco G., and Mercer R. W. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–F650 - PubMed
-
- Cornelius F., and Mahmmoud Y. A. (2003) Functional modulation of the sodium pump: the regulatory proteins “Fixit”. News Physiol. Sci. 18, 119–124 - PubMed
-
- Garty H., and Karlish S. J. (2006) Role of FXYD proteins in ion transport. Annu. Rev. Physiol. 68, 431–459 - PubMed
-
- Geering K. (2006) FXYD proteins: new regulators of Na-K-ATPase. Am. J. Physiol. Renal Physiol. 290, F241–F250 - PubMed
-
- Sweadner K. J., and Rael E. (2000) The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68, 41–56 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

