Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells
- PMID: 26430063
- PMCID: PMC4665007
- DOI: 10.1101/gr.192237.115
Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells
Abstract
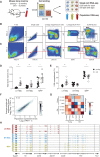
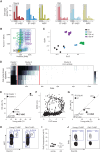
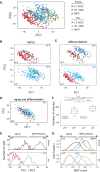
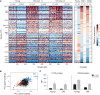
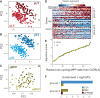
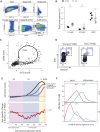
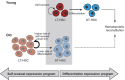
Both intrinsic cell state changes and variations in the composition of stem cell populations have been implicated as contributors to aging. We used single-cell RNA-seq to dissect variability in hematopoietic stem cell (HSC) and hematopoietic progenitor cell populations from young and old mice from two strains. We found that cell cycle dominates the variability within each population and that there is a lower frequency of cells in the G1 phase among old compared with young long-term HSCs, suggesting that they traverse through G1 faster. Moreover, transcriptional changes in HSCs during aging are inversely related to those upon HSC differentiation, such that old short-term (ST) HSCs resemble young long-term (LT-HSCs), suggesting that they exist in a less differentiated state. Our results indicate both compositional changes and intrinsic, population-wide changes with age and are consistent with a model where a relationship between cell cycle progression and self-renewal versus differentiation of HSCs is affected by aging and may contribute to the functional decline of old HSCs.
© 2015 Kowalczyk et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. 2001. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+c-kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15: 659–669. - PubMed
-
- Beghe C, Wilson A, Ershler WB. 2004. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med 116(Suppl 7A): 3S–10S. - PubMed
-
- Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. 2010. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 6: 48–58. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
